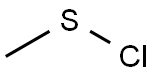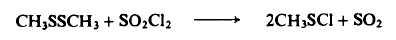Uses
Methanesulfenic acid chloride reacts as a pseudohalogen; reacts with carbanions and enolates to add the thiomethyl group α to the carbonyl; adds across the double bonds of alkenes and allenes; adds across triple bond of alkynes.
Preparation
To a flask containing 23.6 gm (0.25 mole) of dimethyl disulfide at - 1 5 ° to -20°C is slowly added dropwise 34 gm (0.25 mole) of freshly distilled sulfuryl chloride while keeping the temperature at -15° to -20°C. The reaction mixture is stirred for 1 hr, and then distilled to afford 37.1 gm (90%), b.p. 27-28°C (53-60 mm Hg); NMR, 2.91 ppm (singlet). The crude product can also be used without distillation since most of the material is product.
storage
Methanesulfenic acid chloride undergoes decomposition within 1-2 d even when kept in a refrigerator; lasts less than 1 month when stored at -78 °C.
Purification Methods
Methanesulfenic acid chloride can be used crude from the above preparations. If purification is necessary it must be distilled at low temperature, under vacuum, due to its instability.