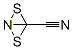Chemical Properties
White to pale yellow crystals. M.p. 15-16°C. At room temperature, explosive conversion to a brick-red, solid material. It is decomposed by water to HCN, HNCS and H2SO4. When SCN- is added to an aqueous (SCN)2 solution, pale yellow (SCN)3 is formed. More electronegative than I2; liberates I2 from iodides. Soluble in alcohol, ether, CS2 and CCl4.
Chemical Properties
The starting materials are NOCl, free of chlorine and nitric oxide, 2 to 4g of which is sealed into ampoules with break-off ends, and anhydrous KNCS, somewhat less than stoichiometrically required for reaction with the NOCl (1 g of NOCl is equivalent to 1.484 g of KNCS). About 25 ml of liquid SO2 per g of NOCl (the SO2 is dried over P2O5) is used as the solvent. The KNCS is introduced into the bottom sphere I, and enough SO2 is condensed to cover the salt with a 1.5-cm layer of liquid. The suspension is then frozen in a liquid-nitrogen bath, and the NOCl and, finally, the remaining SO2 are condensed on top of the frozen layer. The reaction begins immediately after thawing. The apparatus must be occasionally agitated. The reaction temperature should be about -30°C.
After one hour, the resulting deep-red suspension is cooled to -50°C. The apparatus must be immersed in the cooling bath above the level of the fritted glass filter. The product is evaporated by cooling the flask that initially contained the solvent in a liquid-nitrogen bath. The NOSCN product decomposes simultaneously, liberating NO, which is also frozen by the liquid-nitrogen bath. The evaporation must be performed slowly and with care and requires about 10 hours. After the red NOSCN colour has almost entirely disappeared, the same amount of solvent is again condensed on the colourless (SCN)2, and the solution is filtered into sphere II. After another careful evaporation at -30°C, completely colourless, pure (SCN)2 is obtained in 100% yield.