Synthesis
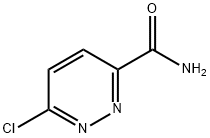
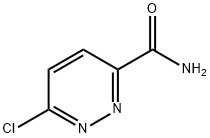
1. An excess of gaseous ammonia was slowly passed into a solution of n-butyl 6-chloropyridazine-3-carboxylate (40 g) in methanol (280 ml) in an ice bath. 2. The reaction mixture was stirred at room temperature for 4 h. 3. Upon completion of the reaction, the solid product was collected by filtration and washed with methanol (20 ml). 4. Drying gave 6-chloropyridazine-3-carboxamide (28.05 g, yield 95.5%) with a melting point of 243-245°C. 5. NMR hydrogen spectroscopy (DMSO-d6) data: δ 7.96 (broad peak, 1H), 8.07 (double peak, 1H, J = 8.3 Hz), 8.22 (double peak, 1H, J = 8.3 Hz), 8.52 (broad peak, 1H). 6-Chloropyridazine-3-carboxamide was dried.
References
[1] Patent: US6100258, 2000, A
[2] Patent: US5843942, 1998, A