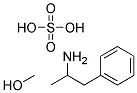
Chemical Properties
Amphetamine is a mobile liquid with an
amine odor.
Chemical Properties
white crystalline powder
Uses
Stimulant (central).
Definition
ChEBI: Amphetamine sulfate is an organic sulfate salt. It contains an amphetamine.
brand name
Benzadrine
(SmithKline Beecham).
General Description
White crystalline powder. Solution in water has a pH of 5-6. Odorless. Slightly bitter taste followed by a sensation of numbness. May be sensitive to light.
Air & Water Reactions
Water soluble. Hygroscopic.
Reactivity Profile
Incompatible with alkalis and calcium salts. . A weak acid.
Fire Hazard
Flash point data for DL-AMPHETAMINE SULFATE--DEA SCHEDULE II ITEM are not available; however, DL-AMPHETAMINE SULFATE--DEA SCHEDULE II ITEM is probably combustible.
Safety Profile
A poison via ingestion,intraperitoneal, and subcutaneous routes. When heated todecomposition it emits very toxic fumes of SOx and NOx.
Safety Profile
Poison by ingestion,subcutaneous, intravenous, and intraperitoneal routes.Human systemic effects by ingestion: altered sleep time,anorexia, and change in heart rate. A central nervoussystem stimulant. An experimental teratogen. Otherexperimental repr
Potential Exposure
Amphetamine is used as a pharmaceutical.
It is a CNS stimulant.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides.
Waste Disposal
It is inappropriate and possibly
dangerous to the environment to dispose of expired or
waste pharmaceuticals by flushing them down the toilet or
discarding them to the trash. Household quantities of
expired or waste pharmaceuticals may be mixed with wet
cat litter or coffee grounds, double-bagged in plastic, discard
in trash. Larger quantities shall carefully take into consideration
applicable DEA, EPA, and FDA regulations. If
possible return the pharmaceutical to the manufacturer for
proper disposal being careful to properly label and securely
package the material. Alternatively, the waste pharmaceutical
shall be labeled, securely packaged and transported by a
state licensed medical waste contractor to dispose by burial
in a licensed hazardous or toxic waste landfill or incinerator
Dissolve or mix the material with a combustible solvent
and burn in a chemical incinerator equipped with an afterburner
and scrubber. All federal, state, and local environmental
regulations must be observed.