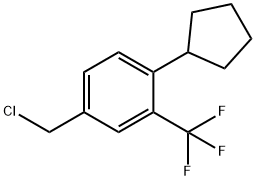
Uses
4-chloromethyl-1-cyclopentyl-2-(trifluoromethyl)benzene is a precursor of APD334, which is a potent functional antagonist of S1P1 and has a favourable PK/PD profile, producing robust lymphocyte lowering at relatively low plasma concentrations in several preclinical species[1].
Synthesis
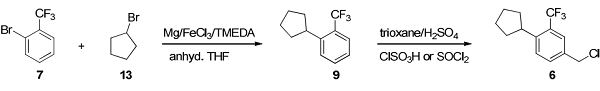
A two-step scalable process was developed starting from commercially available and inexpensive starting materials. An iron(III) chloride-catalyzed aryl–alkyl cross-coupling reaction provided the intermediate 1-cyclopentyl-2-(trifluoromethyl)benzene (9), which was converted to the target building block 4-chloromethyl-1-cyclopentyl-2-(trifluoromethyl)benzene by a direct regioselective chloromethylation reaction with trioxane/thionyl chloride or chlorosulfonic acid in sulfuric acid[2].
References
[1] Daniel J. Buzard*. “Discovery of APD334: Design of a Clinical Stage Functional Antagonist of the Sphingosine-1-phosphate-1 Receptor.” ACS Medicinal Chemistry Letters 5 12 (2014): 1313–1317.
[2] Dipanjan Sengupta. “An Efficient Scale-Up Process for the Preparation of the APD334 Precursor 4-Chloromethyl-1-cyclopentyl-2-(trifluoromethyl)benzene.” Organic Process Research Development 19 6 (2015): 618–623.