Synthesis
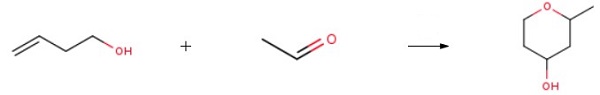
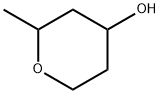
By the procedure of Ernst Hanschke (Chem. Ber., 1955, 88, 1053-1059), the acetaldehyde (1 equiv) and 3-buten-l-ol (1 equiv) are combined with 20% H2SO4 (10 vol) in a pressure tube. The tube was sealed and the reaction mixture was heated to 80 °C for 3 h. Reaction progress is monitored by TLC. After that, the reaction mixture is allowed to cool to rt, carefully neutralized (pH 8-9) by the coned ammonia, and extracted with EtOAc (3 x 5 vol). The combined organic phases are dried (Na2SO4), filtered, and the filtrate coned in vacuo providing the desired product 2-Methyl-tetrahydro-pyran-4-ol.