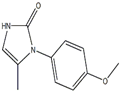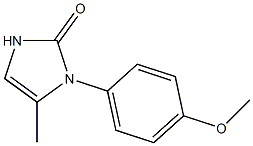
Ranibizumab
- Product NameRanibizumab
- CAS347396-82-1
- CBNumberCB12495883
- MFC11H12N2O2
- MW204.22518
- MOL File347396-82-1.mol
- MSDS FileSDS
Chemical Properties
| form | Liquid |
| color | Colorless to light yellow |
| CAS DataBase Reference | 347396-82-1 |
| FDA UNII | ZL1R02VT79 |
| NCI Drug Dictionary | Lucentis |
| ATC code | S01LA04 |
Ranibizumab Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| Biosynth Carbosynth FR139132 | 1mg | $125 | Ranibizumab - 25mg/ml solution in PBS |
Buy |
| Biosynth Carbosynth FR139132 | 2mg | $200 | Ranibizumab - 25mg/ml solution in PBS |
Buy |
| Biosynth Carbosynth BR171775 | 1mg | $250 | Ranibizumab - 20mg/ml solution in PBS |
Buy |
| Biosynth Carbosynth BR171775 | 2mg | $350 | Ranibizumab - 20mg/ml solution in PBS |
Buy |
| Biosynth Carbosynth FR139132 | 5mg | $400 | Ranibizumab - 25mg/ml solution in PBS |
Buy |
Ranibizumab Chemical Properties,Usage,Production
Description
Ranibizumab is a recombinant, humanized, IgG1 monoclonal antibody fragment (Fab) that neutralizes all active forms of vascular endothelial growth factor A (VEGF-A), and it is indicated for the treatment of neovascular age-related macular degeneration (AMD). It consists of a nonbinding human sequence and a high-affinity binding epitope (Fab fragment) derived from the mouse. The full-length RhuMab VEGF (bevacizumab) was launched previously by Genentech for the treatment of colorectal cancer. Both the antibody fragment and the full-length antibody bind to and inhibit all active forms of VEGF-A and are derived from the same mouse monoclonal antibody. However, ranibizumab has been genetically engineered through a process of selective mutation to increase its affinity for binding and inhibiting the growth factor.Originator
Genentech (US)Uses
Ranibizumab, a humanized antigen binding portion of a murine anti- VEGF monoclonal antibody with a mature high affinity for all VEGF isoforms, has been approved as an intravitreal treatment for neovascular AMD and is the first such treatment to improve visual acuity (VA) in neovascular AMD.CRUISE is multicenter, randomized, double-masked, sham injectioncontrolled Phase III study of 392 participants designed to assess the efficiency and safety profile of ranimizumab for macular edema associated to CRVO. The month 6 result showed that 46.2% (61/132) of patients received 0.3 mg of ranimizumab and 47.7% (62/130) received 0.5 mg of ranimizumab had their vision improved by 15 letters or more compared to 16.9% (22/130) of patients receiving sham injections and mean gain was observed beginning at day seven with an 8.8 and 9.3 letter gain in the 0.3 mg and 0.5 mg study arms of ranimizumab, respectively, compared with 1.1 letters in the sham injection arm.
Uses
antiangiogenic monoclonal antibody macular degeneration therapybrand name
LucentisPreparation Products And Raw materials
Ranibizumab Supplier
Global(52)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +8618092446649 | sarah@tnjone.com | China | 1143 | 58 | |
| +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21631 | 55 | |
| +86-0371-86658258 +8613203830695 |
laboratory@coreychem.com | China | 30231 | 58 | |
| 02768782018 18771942761 |
sales01@whmonad.com | CHINA | 979 | 58 | |
| +86-29-89586680 +86-15129568250 |
1026@dideu.com | China | 22793 | 58 | |
| +86-0571-85134551 | sales@afinechem.com | China | 15350 | 58 | |
| 571-86758373 +8613588754946 |
sales@huarongpharm.com | CHINA | 3148 | 58 | |
| support@targetmol.com | United States | 38630 | 58 | ||
| +86-85511178; +86-85511178; |
peter68@ptchemgroup.com | China | 35425 | 58 | |
| +8618058761490 | info@gihichemicals.com | China | 49984 | 58 |
347396-82-1, RanibizumabRelated Search
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine