Synthesis Reference(s)
The Journal of Organic Chemistry, 42, p. 3109, 1977
DOI: 10.1021/jo00439a001
Synthesis
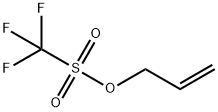
Allyl triflate is prepared by the reaction of Trifluoromethanesulfonic Anhydride (1
equiv) and allyl alcohol (1 equiv) in the presence of pyridine (1 equiv) in carbon tetrachloride at 0 °C.
1 The
insoluble pyridine salt is removed by filtration through sodium sulfate, and the resulting solution is used
directly for allylation reactions. The yield of the reaction is reported to be 75 ± 5% based on quantitative
NMR analysis of aliquots.
storage
Allyl triflate must be stored at -78 °C in a vented flask. The half-life of
the neat reagent is ~10 min at room temperature, and the reagent in carbon tetrachloride solution
completely decomposed in 3 days at room temperature. Allyl triflate reacts violently with nitrogen, or
oxygen-containing solvents such as DMSO, DMF, or acetonitrile. The reagent is an extremely reactive
allylating agent, and contact or inhalation should be avoided. Use in a fume hood.
References
1. Beard, C. D.; Baum, K.; Grakauskas, V. JOC 1973, 38, 3673.