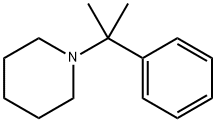
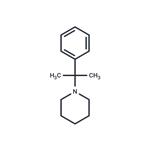
Description
2-Phenyl-2-(1-piperidinyl)propane is an analog of phencyclidine that acts as a mechanism-based inactivator of human cytochrome P450 (CYP) 2B6 (K
i = 5.6 μM; IC
50 = 5.1 μM). It is 15-fold more selective for inhibition of CYP2B6 over CYP2D6 and 40-60-fold more selective for CYP2B6 over CYP1A2, CYP2A6, CYP2Cs, and CYP3A.
Chemical Properties
Colorless to Light Yellow Oil
Uses
Enzyme inhibitor. A selective inactivator of CYP2B6.
in vitro
previous study found that 2-phenyl-2-(1-piperidinyl)propane could inactivate the 7-(benzyloxy)resorufin o-dealkylation activity of liver microsomes obtained from phenobarbital-induced rats. the 7-ethoxy-4-(trifluoromethyl)coumarin o-deethylation activity of purified rat liver p450 2b1 and expressed human p450 2b6 was also inactivated by 2-phenyl-2-(1-piperidinyl)propane in a reconstituted system. with nadph, the loss of activity was founf to be both time- and concentration-dependent, and followed pseudo first order kinetics. the time for 50% of the p450 2b1 to become inactivated at saturating concentrations of 2-phenyl-2-(1-piperidinyl)propane was ~2.5 min. p450 2b6 was inactivated by 2-phenyl-2-(1-piperidinyl)propane with a k(inact) of 0.07 min(-1), a k(i) of 1.2 microm, and a t(1/2) of 9.5 min. the inactivated p450s 2b1 and 2b6 lost about 25 and 15%, respectively, indicating that the loss of activity was caused by a 2-phenyl-2-(1-piperidinyl)propane modification of the apoprotein rather than the heme [1].
References
[1] chun j, kent um, moss rm, sayre lm, hollenberg pf. mechanism-based inactivation of cytochromes p450 2b1 and p450 2b6 by 2-phenyl-2-(1-piperidinyl)propane. drug metab dispos. 2000 aug;28(8):905-11.