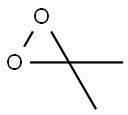
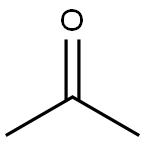
Synthesis
A 2-L, three-necked, round-bottomed flflask containing a mixture of water (80 mL), acetone (50 mL, 0.68 mol), and sodium bicarbonate (96 g) is equipped with a magnetic stir bar and a pressure equalizing addition funnel containing water (60 mL) and acetone (60 mL, 0.82 mol). A solid addition flflask containing Oxone (180 g, 0.29 mol) is attached to the reaction vessel via a rubber tube. An air condenser (20 cm length) loosely packed with glass wool is attached to the reaction vessel. The outlet of the air condenser is connected to a 75 × 350-mm Dewar condenser fifilled with dry ice–acetone that is connected to a receiving flflask (100 mL) cooled in a dry ice–acetone bath. The receiving flflask is also connected in series to a second dry ice–acetone cold trap, a trap containing a potassium iodide solution, and a drying tube. A gas inlet tube is connected to the reaction flflask and a stream of nitrogen gas is bubbled through the reaction mixture. The Oxone is added in portions (10–15 g) while the acetone–water mixture is simultaneously added dropwise. The reaction mixture is stirred vigorously throughout the addition of reagents (approximately 30 min). A yellow solution of dimethyldioxirane in acetone collects in the receiving flflask. Vigorous stirring is continued for an additional 15 min while a slight vacuum (about 30 mm, water aspirator) is applied to the cold trap. The yellow dioxirane solution (62–76 mL) is dried over sodium sulfate (Na2SO4), fifiltered, and stored in the freezer (–25°C) over Na2SO4. The dioxirane content of the solution is assayed using phenyl methyl sulfifide and the gas-liquid chromatography (GLC) method. Generally, concentrations in the range of 0.07–0.09 M are obtained.
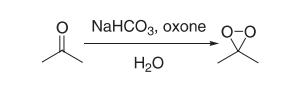
Reference: Murray, R. W.; Singh M. Org. Syn. 1998, Coll. Vol. IX, 288−293.
storage
Solutions of the dimethyldioxirane can be kept in the freezer of a refrigerator (-10 to -20 °C) for as long as a week. The concentration of the reagent decreases relatively slowly, provided solutions are kept from light and traces of heavy metals. These dilute solutions are not known to decompose violently, but the usual precautions for handling peroxides should be applied, including the use of a shield. All reactions should be performed in a fume hood to avoid exposure to the volatile oxidant.