Description
The widespread efficacy of opioids in treating patients with moderate to
severe acute and chronic pain is often accompanied by untoward side
effects. In particular, opioid-induced bowel dysfunction is one of the
more common and debilitating consequences afflicting up to 50% of
patients. To counteract the peripherally-mediated adverse effects, opioid
antagonists such as naloxone, naltrexone, and nalmephene are sometimes prescribed. The latest market entry exploits a strategic
modification of naltrexone to lower its lipid solubility and increase its
polarity: quaternization of the amine of naltrexone by methylation
(methyl bromide) prevents crossing of the blood–brain barrier thereby
creating an effective peripheral antagonist. Despite a loss of potency
upon methylation, methylnaltrexone antagonizes opioid binding at
m-opioid receptors with an IC
50 of 70 nM. Its affinity for k-opioid receptors
is approximately eightfold less (IC
50= 575 nM) with no significant binding
to d-opioid, orphanin, or non-opioid receptors. Methylnaltrexone bromide
has been approved for the treatment of opioid-induced constipation in
patients with advanced illness receiving palliative care.Regarding metabolism, methylnaltrexone bromide is eliminated primarily as intact drug (85% based on administered radioactivity) by slightly more renal than hepatic clearance.
The most common adverse events were abdominal pain and flatulence followed by nausea, dizziness, and diarrhea.
Chemical Properties
Pale Pink Solid
Originator
University of Chicago (United States)
Uses
A metabolite of Naltrexone (N285750). Methylnaltrexone (MNTX), a selective μ-opioid receptor antagonist, functions as a peripherally acting receptor antagonist in tissues of the gastrointestinal tract.
Uses
Methylnaltrexone bromide has been used as a drug to measure plasma protein binding (PPB), permeability (Pm) and the membrane coefficient (KIAM) for the prediction of blood brain barrier (BBB) penetration. It is also used as a mu-opioid receptor (MOR) antagonist to abrogate morphine tolerance and opioid-induced hyperalgesia (OIH).
General Description
Methylnaltrexone does not cross blood brain barrier and does not affect the opioid effects in the brain, such as analgesia. It is used to treat opioid-induced constipation (OIC).
Biochem/physiol Actions
Methylnaltrexone bromide is a narcotic antagonist. It is a peripheral mu-opiod receptor antagonist that cannot cross the blood-brain barrier. It reverses many opioid side-effects without interfering with pain relief.
Clinical Use
N-Methylnaltrexone
is the polar derivative
of naltrexone, which does not reach the central
nervous system but can block intestinal opioid
receptors. The compound is under development
to counteract opioid induced bowel dysfunction
such as constipation and megacolon .
Synthesis
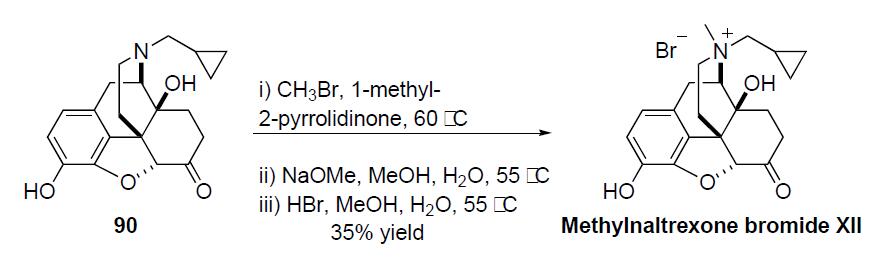
The
synthesis of methylnaltrexone bromide proceeds in a
straightforward manner via the alkylation of naltrexone 90
in the following scheme. Naltrexone 90 was reacted with methyl
bromide in 1-methyl-2-pyrrolidinone at 60 ??C. The resulting
crude product was treated with sodium methoxide in methanol/
water at 55 ??C to remove any undesired phenolic (Oalkylated)
side-products. The resulting crude sodium salt was
treated with hydrobromic acid in methanol/water and upon
crystallization gave methylnaltrexone bromide (XII) in 35%
overall yield.