Uses
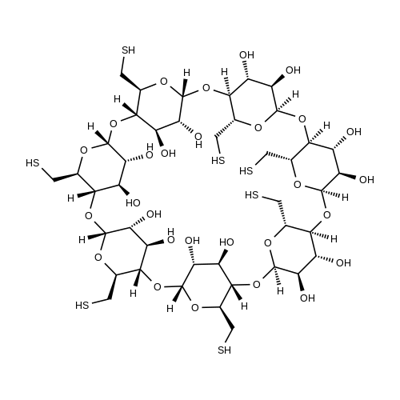
Heptakis-(6-Mercapto-6-deoxy)-beta-Cyclodextrin(Cas#160661-60-9)is a modified beta-cyclodextrin as a reagent in lab& research institution. Sulphur is lively group by oxidation or replace to create higher levels of supramolecular structure.Mercaptoacetic extremely easy adherent to gold surface, thus realize novel applications.Mercaptoacetic cyclodextrins derivatives extremely suitable for drug induction of anesthesia neuromuscular reversal.
Synthesis
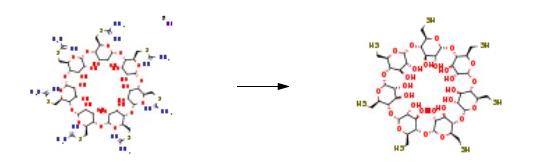
The thiuronium salt (12.2 g, 0.005 mol) was heated around 100 ??C in deoxygenated (He/ultrasound) 0.25 M NaOH (120 mL) for 4 h. As the reaction was completed the reaction mixture was cooled to around 5-10 ??C and the pH was adjusted to 1.0-1.5 with 3 M HCl solution, when the product started to crystallize. As the pH became stable, the suspension was allowed to crystallize for 15 min, the solids were removed by filtration, washed with deoxygenated deionized water to around pH 5-6, then dried in vacuo at rt in the presence of P2O5 and KOH, and yielded 6.5 g of dark orange, glassy crystals. The obtained product was recrystallized by dissolution in deoxygenated 1 M NaOH solution (12 mL) and precipitated with 1 M HCl (18 mL). Filtration and washing to neutral pH with deoxygenated water afforded 3.8 g (61% theor.) light orange crystals. RF = 0.00-0.05 10:7 dioxane/cc. NH3.3.5. Regeneration of thiol group from its oxidized statePartially oxidized heptakis(6-deoxy-6-thio)cyclomaltoheptaose (6.2 g, approx. 0.010 mol) was suspended in deoxygenated water and deoxygenated (He/ultrasound) 3 M NaOH solution (30 mL) was added at rt. Sodium borohydride (1.1 g, 0.030 mol) is added and stirred overnight. The occasionally found solids were filtered through a sintered glass funnel, the filtrate was cooled with an ice-water bath and the pH was adjusted to around 1.5-2 with 3 M HCl solution (approx. 45 mL). Upon acidification the product started to crystallize, and as the pH became stable and foaming stopped, the suspension was allowed to crystallize for an additional 15 min. The product was removed by filtration and washed to pH 5-7 with deoxygenated deionized water, then dried in vacuo at rt in the presence of P2O5 and KOH, yielded 3.2 g (50%) |?-CDSH as a yellowish substance.