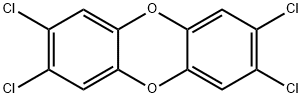
2,3,7,8-Tetrachlordibenzo[b,e][1,4]dioxin
Bezeichnung:2,3,7,8-Tetrachlordibenzo[b,e][1,4]dioxin
CAS-Nr1746-01-6
Englisch Name:2,3,7,8-TETRACHLORODIBENZO-P-DIOXIN
CBNumberCB6739638
SummenformelC12H4Cl4O2
Molgewicht321.97
MOL-Datei1746-01-6.mol
Synonyma
2,3,7,8-Tetrachlordibenzo[b,e][1,4]dioxin
2,3,7,8-TCDD
2,3,7,8-Tetrachlor-1,4-dioxin
2,3,7,8-Tetrachlordibenzo[b,e][1,4]dioxin physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 284-287°C |
| Siedepunkt | 407.62°C (rough estimate) |
| Dichte | 1.6430 (estimate) |
| Dampfdruck | 340 at 25 °C (Rodorf, 1985) |
| Brechungsindex | 1.6430 (estimate) |
| Flammpunkt | 4 °C |
| storage temp. | Refrigerator |
| Löslichkeit | Chloroform: Slightly soluble |
| Aggregatzustand | Colorless solids or crystals |
| Farbe | Colorless to white needles |
| Wasserlöslichkeit | 0.0193ug/L(22 ºC) |
| Henry's Law Constant | 5.40 at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Expositionsgrenzwerte | An IDLH of 1 ppb was recommended by Schroy et al. (1985). |
| CAS Datenbank | 1746-01-6(CAS DataBase Reference) |
| IARC | 1 (Vol. Sup 7, 69, 100F) 2012 |
| EPA chemische Informationen | 2,3,7,8-Tetrachlorodibenzo-p-dioxin (1746-01-6) |
| Kennzeichnung gefährlicher | F,Xn |
| R-Sätze: | 11-38-48/20-63-65-67 |
| S-Sätze: | 36/37-62-46 |
| RIDADR | 2811 |
| WGK Germany | 3 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| Giftige Stoffe Daten | 1746-01-6(Hazardous Substances Data) |
| Toxizität | LD50 in male, female rats (mg/kg): 0.022, 0.045 orally (Schwetz) |




