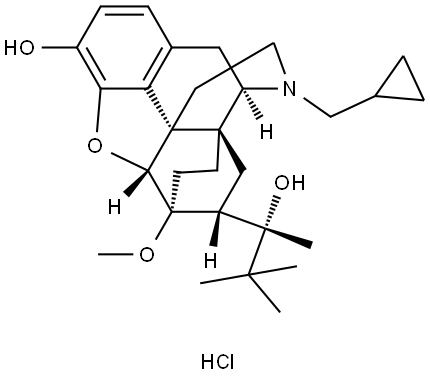
[5α,7α(S)]-α-tert-Butyl-17-(cyclopropylmethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-α-methyl-6,14-ethenomorphinan-7-methanolhydrochlorid
Bezeichnung:[5α,7α(S)]-α-tert-Butyl-17-(cyclopropylmethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-α-methyl-6,14-ethenomorphinan-7-methanolhydrochlorid
CAS-Nr53152-21-9
Englisch Name:BUPRENORPHINE HYDROCHLORIDE
CBNumberCB5140046
SummenformelC29H41NO4.ClH
Molgewicht504.1
MOL-Datei53152-21-9.mol
Synonyma
[5α,7α(S)]-α-tert-Butyl-17-(cyclopropylmethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-α-methyl-6,14-ethenomorphinan-7-methanolhydrochlorid
[5α,7α(S)]-α-tert-Butyl-17-(cyclopropylmethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-α-methyl-6,14-ethenomorphinan-7-methanolhydrochlorid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 260-262?C (dec.) |
| storage temp. | -0°C |
| Löslichkeit | Sparingly soluble in water, freely soluble in methanol, soluble in ethanol (96 per cent), practically insoluble in cyclohexane. |
| Aggregatzustand | Powder |
| Wasserlöslichkeit | Soluble to 25 mM in water and to 50 mM in ethanol |
| Kennzeichnung gefährlicher | Xn,T,F |
| R-Sätze: | 22-62-63-39/23/24/25-23/24/25-11 |
| S-Sätze: | 26-36-45-36/37-16-7 |
| WGK Germany | 3 |
| RTECS-Nr. | KM7758000 |
| HS Code | 2939110000 |


