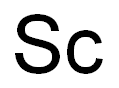
Scandium
Bezeichnung:Scandium
CAS-Nr7440-20-2
Englisch Name:SCANDIUM
CBNumberCB4115641
SummenformelSc
Molgewicht44.96
MOL-Datei7440-20-2.mol
Synonyma
Scandium
Scandium physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 1540 °C (lit.) |
| Siedepunkt | 2836 °C (lit.) |
| Dichte | 2.99 g/mL at 25 °C (lit.) |
| storage temp. | 15-25°C |
| Löslichkeit | Slowly dissolve in dilute acids. |
| Aggregatzustand | powder |
| Farbe | Silver-gray |
| Wichte | 3 |
| Flame Color | Orange |
| Widerstand (resistivity) | 50.5 μΩ-cm, 0°C |
| Wasserlöslichkeit | Reacts with water. |
| Sensitive | air sensitive |
| Merck | 13,8468 |
| Expositionsgrenzwerte | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| InChIKey | SIXSYDAISGFNSX-UHFFFAOYSA-N |
| CAS Datenbank | 7440-20-2(CAS DataBase Reference) |
| EPA chemische Informationen | Scandium (7440-20-2) |
| Kennzeichnung gefährlicher | F,T,Xi |
| R-Sätze: | 5-11-34-23/24/25-36/37/38-36/38 |
| S-Sätze: | 26-28-36/37-7/9-36/37/39-16-43-45-27 |
| RIDADR | UN 3089 4.1/PG 2 |
| WGK Germany | 1 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 2805304000 |

