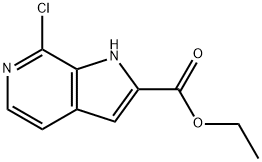
To a suspension of potassium ethyl ether (6.56 g, 77.9 mmol) in ether (55 mL) was slowly added diethyl oxalate (10.6 mL, 77.9 mmol), the reaction was slightly exothermic. Stirring for 5 minutes resulted in a homogeneous yellow solution, which transformed to an inhomogeneous yellow slurry after 10 minutes. 2-Chloro-4-methyl-3-nitropyridine (13.45 g, 77.9 mmol) solid was added and washed with ether (23 mL) to give a dark purple solution containing a dark precipitate. The mixture was stirred at room temperature for 21 hours. The solid precipitate was collected by filtration, washed thoroughly with ether and dried over air to afford (LZ)-1-(2-chloro-3-nitropyridin-4-yl)-3-ethoxy-3-oxoprop-1-en-2-ol potassium salt (19.8 g, 63.6 mmol, 81% yield) as an orange solid, which could be used for the next reaction without further purification. The above potassium salt (19.8 g, 63.6 mmol) was dissolved in acetic acid (908 mL) and iron powder (14.6 g, 280.9 mmol) was added. The reaction mixture was heated to 60 °C and stirred for 18.5 h. TLC analysis showed complete consumption of the feedstock and the reaction mixture was filtered through diatomaceous earth to remove the catalyst. The filtrate was concentrated to dryness and the residue was treated with dichloromethane (~400 mL) and filtered through a silica gel column. The insoluble material was removed by elution with dichloromethane, followed by dichloromethane/ethyl acetate (50:50), and concentration afforded ethyl 7-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate (10.3 g, 45.8 mmol, 72% yield) as a yellow solid: Rf 0.80 (silica gel, 50:50 hexane/ethyl acetate); melting point 152-157 °C; 1H NMR (300MHz, CD3OD) δ 1.43 (3H, t, J=7.0Hz), 4.44 (2H, q, J=7.1Hz), 7.27 (1H, s), 7.65 (1H, d, J=5.7Hz), 7.95 (1H, d, J=5.4Hz); ESI MS m/z 224 [C10H9ClN2O2 +H]+; HPLC (Method A) >99% (AUC), tR=16.6 min. Ethyl 7-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate (0.64 g, 2.85 mmol) was dissolved in tetrahydrofuran (5.7 mL) and methanol (6.8 mL) and 3N KOH (2.85 mL) was added. After stirring at room temperature for 15.5 hours, the reaction mixture was concentrated to dryness. The residue was dissolved in water, adjusted to pH 3 with 6N HCl and the precipitate was collected by filtration. The precipitate was dissolved in methanol and concentrated to dryness to give 7-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylic acid (0.53 g, 2.7 mmol, 94% yield) as a yellow powder: melting point 210-214 °C; 1H NMR (300 MHz, CD3OD) δ 7.25 (1H, s), 7.65 (1H, d, J=5.4 Hz), and 7.94 (1H, d, J=5.4Hz); ESI MS m/z 195 [C8H5ClN2O2-H]-; HPLC (Method A) >99% (AUC), tR=12.2 min.