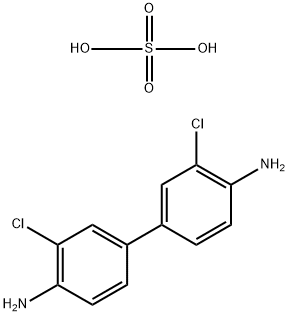
General Description
A white crystalline powder. Toxic by ingestion and skin absorption.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
A halogenated acidic organic salt. Materials in this group are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Potential Exposure
A halide-and amine-substituted aromatic compound used in the dye industry, curing agent for
isocyanate terminated resins. The major uses of dichlorobenzidine are in the manufacture of pigments for printing
ink, textiles, plastics, and crayons and as a curing agent
for solid urethane plastics. There are no substitutes for
many of its uses. Additional groups that may be at risk
include workers in the printing or graphic arts professions
handling the 3,3’-DCB-based azo pigments. 3,3’-DCB may
be present as an impurity in the pigments, and there
is some evidence that 3,3’-DCB may be metabolically
liberated from the azo pigment
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR
if heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get
medical attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required
Incompatibilities
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, and epoxides. Achemical base: neutralize acids to form salts plus water with an exothermic
reaction. May be incompatible with isocyanates, halogenated
organics, peroxides, phenols (acidic), epoxides, anhydrides,
and acid halides. Flammable gaseous hydrogen is generatedby amines in combination with strong reducing agents such
as hydrides, nitrides, alkali metals, and sulfides.
Waste Disposal
Incineration (816�C,
0.5 second for primary combustion; 1204�C, 1.0 second for
secondary combustion). The formation of elemental
chlorine can be prevented through injection of steam ormethane into the combustion process. nitrogen oxides
may be abated through the use of thermal or catalytic
devices. Consult with environmental regulatory agencies
for guidance on acceptable disposal practices. Generators
of waste containing this contaminant (≥100 kg/mo) must
conform with EPA regulations governing storage, transportation, treatment, and waste disposal