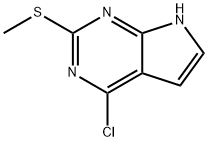
General procedure for the synthesis of 4-chloro-2-(methylthio)-7H-pyrrolo[2,3-d]pyrimidines from 2-methylthio-4-hydroxy-7H-pyrrolo[2,3-d]pyrimidines: triclosan (5 mL, 53.7 mmol) and triethylamine (0.2 mL, 1.43 mmol) were added to 2-methylthio-7H-pyrrolo[2,3-d]pyrimidin-4-yl alcohol (0.10 g, 0.55 mmol). The reaction mixture was heated to reflux at 130 °C for 4 h and subsequently cooled to room temperature. Excess trichlorophosphate was removed by distillation under reduced pressure. Ice water was carefully added to the residue and the suspension was extracted with ether (3 x 20 mL). The organic phases were combined, washed with distilled water (10 mL), dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography (Florisil, 100-200 mesh), using diethyl ether as eluent, to give the pure 4-chloro-2-methylthio-7H-pyrrolo[2,3-d]pyrimidine in 77% yield (literature value 43%). Melting point: 207-209°C. 1H NMR (CDCl3): δ 2.67 (s, 3H, SCH3), 6.56-6.59 (m, 1H, H-5), 7.21-7.23 (m, 1H, H-6). IR (KBr) cm-1: 3412 (NH), 3122 (CH), 1614 (C=C). Mass Spectrometry (MS): m/z [M+1]+ 201. Calculated values for elemental analysis (C7H6N3ClS): C 42.11, H 3.03, N 21.05, S 16.06; measured values: C 42.29, H 3.38, N 21.14, S 16.36.
![2-METHYLSULFANYL-7H-PYRROLO[2,3-D]PYRIMIDIN-4-OL](/CAS/GIF/67831-83-8.gif)
![5-chloro-3-methylsulfanyl-2,4,9-triazabicyclo[4.3.0]nona-2,4,7,10-tetraene](/CAS/GIF/57564-94-0.gif)