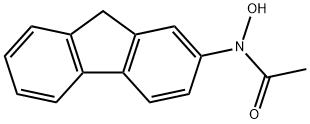
Chemical Properties
2-Acetylaminofl uorene is frequently used in the laboratory by biochemists and technicians
as a positive control in the study of liver enzymes and the carcinogenicity and mutagenicity
of aromatic amines. Exposure to 2-acetylaminofl uorene may occur via inhalation
or dermal contact in laboratories where it is being used in the study of carcinogenesis.
Occupations at greatest risk of exposure are organic chemists, chemical stockroom workers,
and biomedical researchers.
Chemical Properties
cream powder
General Description
Cream colored powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Hydroxyacetylaminofluorene is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition Hydroxyacetylaminofluorene emits toxic fumes of NOx.
Fire Hazard
Flash point data for Hydroxyacetylaminofluorene are not available; however, Hydroxyacetylaminofluorene is probably combustible.
Health Hazard
There is no complete information about the acute or chronic health effects of
2-acetylaminofl uorene in laboratory animals or in humans. However, oral exposures of
2-acetylaminofl uorene in laboratory mice caused moderate acute toxicity.
Uses
N-9H-Fluoren-2-yl-N-hydroxy-acetamide inactivates human arylamine N-acetyltransferases (NATs), NAT1 and NAT2. Mutagenicity examined in Salmonella typhimurium TA1538. Liver produced metabolite of the cancinogen 2-Acetamidofluorene (A158535), which is also a highly carcinogenic compound.
Definition
ChEBI: N-hydroxy-2-acetamidofluorene is a member of 2-acetamidofluorenes.