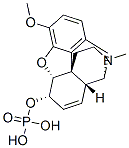
Chemical Properties
White to Off-White Solid
Uses
Present in opium from 0.7% to 2.5%, depending on the source. Weak narcotic analgesic, perhaps due to conversion to morphine, with minimal hypnotic properties; potent antitussive.
Controlled substance (opiate)
General Description
Colorless small crystals or white powder. Odorless. Bitter taste. Solutions are acid to litmus. pH (4% solution) 4.2-5.
Air & Water Reactions
Water soluble.
Reactivity Profile
codeine phosphate is sensitive to exposure to light. . Materials that are acidic salts are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Fire Hazard
Flash point data for codeine phosphate are not available; however, codeine phosphate is probably combustible.
Brand name
Ambenyl
Cough Syrup (Parke-Davis); Colrex Compound (Solvay
Pharmaceuticals).