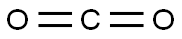Synthesis
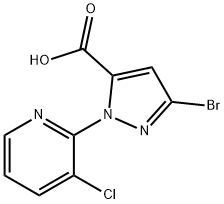
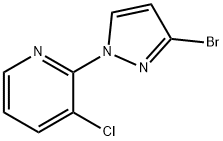
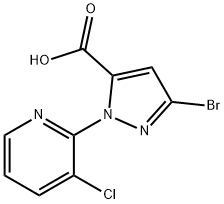
At -78 °C, 2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine (3.04 g, 11.8 mmol) was dissolved in anhydrous THF (25 mL), keeping the reaction temperature below -71 °C. LDA solution (6 mL, 2M) was added slowly and dropwise. The reaction mixture was stirred at -78 °C for 15 min and then bubbled with carbon dioxide gas for 10 min. Subsequently, the reaction system was slowly warmed to -57°C and continued to -20°C. Upon completion of the reaction, the reaction was quenched with water. The reaction mixture was concentrated and dissolved in water (100 mL) and ether (80 mL), and an aqueous NaOH solution (1N, 2 mL) was added. The aqueous phase was washed with ether and acidified with aqueous HCl (2N). The precipitated solid was filtered, washed with water and dried to give 3-bromo-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxylic acid (2 g, 59% yield) as a brown solid.
References
[1] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 22, p. 6274 - 6279
[2] Patent: CN105669643, 2016, A. Location in patent: Paragraph 0072; 0075; 0076
[3] Patent: WO2006/68669, 2006, A1. Location in patent: Page/Page column 19; 25
[4] Patent: WO2003/106427, 2003, A2. Location in patent: Page 18-19
[5] Patent: WO2004/67528, 2004, A1. Location in patent: Page 27