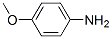Chemical Properties
Anisidine exists as ortho-, meta-, and paraisomers.
They have characteristic amine (fishy) odors.
General Description
A fused crystalline solid or a reddish to yellowish oily liquid. More dense than water and insoluble in water. Very irritating to skin, eyes, and mucous membranes. Toxic by ingestion and skin contact. Used to make dyes.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
An amine. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Potential Exposure
Anisidines are used in the manufacture
of azo dyes; pharmaceuticals; textile-processing chemicals
Incompatibilities: Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. Attacks some coatings
and some forms of plastic and rubber.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR
if heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Shipping
UN2431 Anisidines, Hazard Class: 6.1; Labels:
6.1-Poisonous materials
Waste Disposal
Dissolve in combustible solvent
(alcohols, benzene, etc.) and spray solution into furnace
equipped with afterburner and scrubber, or burn spill
residue on sand and soda ash absorbent in a furnace.
Definition
ChEBI: P-anisidine is a substituted aniline that is aniline in which the hydrogen para to the amino group has been replaced by a methoxy group. It is used as a reagent for the detection of oxidation products such as aldehydes and ketones in fats and oils. It has a role as a reagent and a genotoxin. It is a member of methoxybenzenes, a substituted aniline and a primary amino compound.