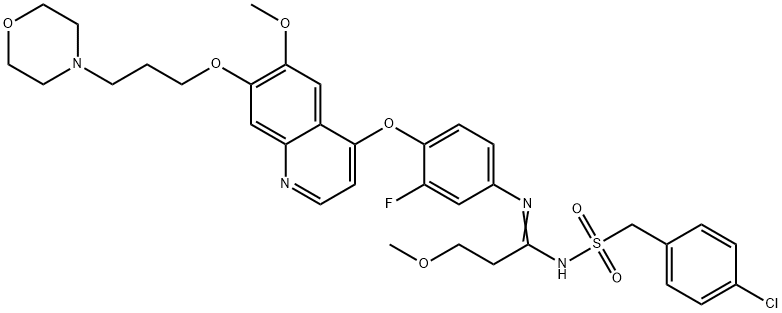
c-Met-IN-14 (compound 26af) (p.o.; 8 mg/kg) exhibits safety profile and favorable pharmacokinetic properties in BALB/c mouse, with rapid absorption (Tmax=2.5 h), high maximum concentration (Cmax=1228.4 ng/mL), high plasma exposure (AUC0-∞=6.8 μg.h.mL-1), accepted elimination half-life (T1/2=3.5 h), and well clearance (1.18 L.h-1.kg-1), has a moderate oral bioavailability (74%) in mouse[1].
c-Met-IN-14 (i.p.; below 200 mg/kg) doesn’t cause abnormalities, anaphylactic responses, allergic reactions on mice[1].
| Animal Model: | 8-week-old male BALB/c mice [1] |
| Dosage: | 0 (vehicle), 100, 200, 300, or 400 mg/kg |
| Administration: | Intraperitoneal injection; treatment on day 0 and assessment every 3 days for 15 days |
| Result: | Showed no obvious toxicity in acute toxicity tests. |
| Animal Model: | Pharmacokinetic profiles of compound 26af in BALB/c mouse[1]
|
| Dosage: | |
| Administration: | |
| Result: | | Route | Dose (mg/kg) | T1/2 (h) | Cmax (ng.mL-1) | Tmax (h) | AUC0-∞ (μg.h.mL-1) | CL (L.h-1.kg-1) | CL (%) | | i.v. | 2 | 1.8 | 675.6 | - | 2.3 | - | | | p.o. | 8 | 3.5 | 1228.4 | 2.5 | 6.8 | 1.18 | 74 |
|