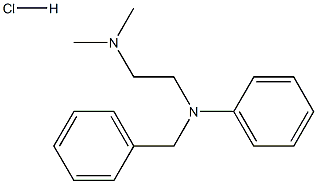
Chemical Properties
beige crystalline powder
General Description
Beige crystals. pH (25 mg/ml solution) 5.72.
Air & Water Reactions
Slightly water soluble. Aqueous solutions are weakly acidic.
Reactivity Profile
Acidic organic/inorganic salts, such as Phenbenzamine hydrochloride, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
SYMPTOMS: On ingestion, Phenbenzamine hydrochloride will cause drowsiness and general central nervous system effects.
Fire Hazard
The flash point of Phenbenzamine hydrochloride has not been determined. Phenbenzamine hydrochloride is probably combustible.
Definition
ChEBI: Phenbenzamine hydrochloride is an organic molecular entity.
Safety Profile
Poison by ingestion, intravenous, and subcutaneous routes. When heated to decomposition it emits very toxic fumes of HCl and NOx. Used as an antihistaminic agent.