Chemical Properties
solid
General Description
Odorless colorless powder or white crystals. Used as a selective pre- and early post-emergence herbicide in soybeans, strawberries, various vegetable crops and ornamentals. Root- and foliage-absorbed herbicide selective in leek, celery, onion, carrot and strawberry.
Reactivity Profile
Fire may produce irritating or poisonous gases. When heated to decomposition CHLOROXURON emits very toxic fumes of chlorides and nitrogen oxides. [EPA, 1998].
Health Hazard
This is highly toxic by ingestion. Under certain conditions, CHLOROXURON will form carcinogenic dimethylnitrosamine.
Fire Hazard
(Non-Specific -- Pesticide, Solid, n.o.s.) Fire may produce irritating or poisonous gases. When heated to decomposition CHLOROXURON emits very toxic fumes of chlorides and nitrogen oxides.
Potential Exposure
A potential danger to those involved in the manufacture, formulation, and application of chloroxuron for use as a selective pre- and early postemergency herbicide in soybeans, strawberries; various vegetable crops, and ornamentals. It is a root- and foliageabsorbed herbicide selective in leek, celery, onion, carrot, and strawberry.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a Medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit
Shipping
UN2767 Phenylurea pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Description
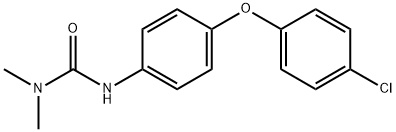
Chloroxuron is a combustible, colorless crystalline solid. Molecular weight =290.77; Freezing/Meltingpoint =15.5℃; Vapor pressure =3.9 x10 -9 mmHg at20℃. Practically insoluble in water.
Waste Disposal
Incinerate in a unit with effluent gas scrubbing. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Definition
ChEBI: Chloroxuron is a member of the class of phenylureas that is N,N-dimethylurea in which a hydrogen of the amino group is replaced by a 4-(4-chlorophenoxy)phenyl group. It is a phenylurea herbicide used for the control of annual grasses, mosses and broad-leaved weeds. Common crop plants for which the herbicicide is useful are soy beans, onions, strawberries, and celery. It has a role as a herbicide. It is an aromatic ether, a member of phenylureas and a member of monochlorobenzenes.
Environmental Fate
Soil. Hartley and Kidd (1987) reported 4-(4-chlorophenoxy)aniline as a soil metabolite.
Chloroxuron was degraded by microorganisms in humus soil and a sandy loam to form
N′-(4-chlorophenoxy)phenyl-N-methylurea, N′-(4-chloro-phenoxy)phenylurea and (4-
chlorophenoxy)aniline and two minor unidentified compounds (Geissbühler et al., 1963a).
Residual activity in soil is limited to approximately 4 months (Hartley and Kidd, 1987).
Plant. In plants, chloroxuron is degraded to monomethylated and demethylated derivatives followed by decarboxylation forming 4-(4-chlorophenoxy)aniline (Humburg et al.,
1989)
Photolytic. The UV irradiation of an aqueous solution of chloroxuron for 13 hours
resulted in 90% decomposition of the herbicide. Products identified (% yield) were mono-
(2.2%) and didemethylated (4.2%) products and carbon dioxide (64%) (Plimmer
Chemical/Physical. Hydrolyzes in strong acids and bases forming 4-(4-chloro-phenoxy)aniline (Hartley and Kidd, 1987). Emits toxic fumes of nitrogen oxides, cyanides
and chlorine when heated to decomposition (Sax and Lewis, 1987)
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with chloroxuron you should be trained on its proper handling and storage. Store in tightly closed containers in a cool, wellventilated area.