Description
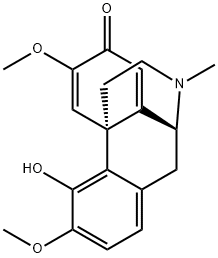
An alkaloid obtained from Croton salutaris, this base has been postulated as an intermediate compound in the biosynthesis of the morphine alkaloids. It yields colourless rods when crystallized from AcOEt and has [α]D
+ 111 ° (c 1.69, EtOH). The ultraviolet spectrum in EtOH has two absorption maxima at 240 and 277 mJl. Two methoxyl groups, a phenolic hydroxyl group and a methylimino group are present, the base furnishing an O-acetate, m.p. 171°C; [α]D + 120° (c 1.25, EtOH). More recently, it has been discovered in Croton balsamifera Jacq. and Papaver orientale.
Uses
Salutaridine is one of the biosynthetic precursors of morphine (M652290), a principle alkaloid of opium.
Definition
ChEBI: Salutaridine is a morphinane alkaloid from the opium poppy, in which the 5,6,8,14-tetradehydromorphinan-7-one skeleton is substituted at position 4 by a hydroxyl group, positions 3 and 6 by methoxy groups and position N17 by a methyl group. An intermediate in the biosynthesis of narcotic analgesics such as morphine and codeine. It has a role as a metabolite and an anti-HBV agent. It is a conjugate base of a salutaridinium(1+). It derives from a hydride of a morphinan.
References
Barton et al., J. Chem. Soc., 2423 (1965)
Battersby, Brown., Chem. Commun., 170 (1966)
Chambers, Haynes, Stuart., ibid, 449 (1966)
Mass spectra:
Wheeler, Kinstle, Rinehart.,J. Amer. Chem. Soc., 89,4494 (1967)