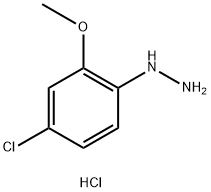
The general procedure for the synthesis of (4-chloro-2-methoxyphenyl)hydrazine hydrochloride from 4-chloro-2-methoxyaniline is as follows: 4-chloro-2-methoxyaniline (1.69 g, 10.7 mmol, 1 eq.) was suspended in a mixture of concentrated hydrochloric acid (25 mL) and acetic acid (10 mL) at 0 °C. A solution of sodium nitrite (740 mg, 10.7 mmol, 1 equiv) in water (10 mL) was added slowly and dropwise. The reaction mixture was gradually warmed to 60 °C and maintained at this temperature for 1.5 hours. Subsequently, the reaction solution was cooled to 0 °C and a concentrated hydrochloric acid (25 mL) solution of stannous chloride (5.32 g, 23.6 mmol, 2.2 equiv) was added. After 30 min of reaction, the precipitate was collected by filtration to afford the target product (4-chloro-2-methoxyphenyl)hydrazine hydrochloride (2.06 g, 93% yield). The product was characterized by 1H NMR (DMSO-d6): δ 9.94 (br.s, 2H), 7.72 (br.s, 1H), 7.07 (s, 1H), 7.00 (s, 2H), 3.85 (s, 3H). With reference to the above method, other intermediates of similar structure can be prepared by substituting appropriate reagents, raw materials and purification methods.