Chemical Properties
Silver Hydroxide (AgOH) is a highly thermally unstable compound which tends to decompose into silver oxide (Ag2O) and water (H2O) when exposed to air or direct light.
Preparation
Silver Hydroxide (AgOH) can be produced by reacting sodium hydroxide with silver nitrate. When sodium hydroxide diffuses in a gel medium containing silver nitrate, silver hydroxide precipitates and forms a white band in a narrow glass tube. Since silver hydroxide is unstable in excess diffused electrolytes, the silver hydroxide precipitate turns into brown silver oxide[1].
Reactions
Silver Hydroxide (AgOH) reacts with acids to form soluble silver salts. For example, when AgOH reacts with concentrated hydrochloric acid HCl, silver chloride (AgCl) and water are formed. The reaction formula is as follows:
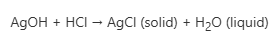
References
[1] LAYLA BADR; Irving E. Propagation behavior of silver hydroxide precipitate bands[J]. Chemical Physics Letters, 2022. DOI:10.1016/j.cplett.2022.139681.

