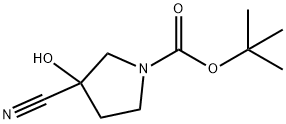
Intermediate: tert-butyl 3-cyano-3-hydroxypyrrolidine-1-carboxylate Synthesis Procedure: to a solution of tert-butyl 3-oxopyrrolidine-1-carboxylate (1 g, 5.40 mmol) in dichloromethane (DCM, 10 mL) at 0 °C was added sequentially trimethylmethylsilyl cyanide (TMSCN, 0.724 mL), potassium cyanide (KCN, 0.035 g 0.540 mmol), and 18-crown-6 (0.143 g, 0.540 mmol). The reaction mixture was slowly warmed to room temperature and stirred continuously for 16 hours. After completion of the reaction, the mixture was cooled to 0 °C and the reaction was quenched with saturated aqueous sodium bicarbonate (NaHCO3). Subsequently, the reaction mixture was diluted with ethyl acetate (EtOAc), the organic phase was separated and washed with saturated aqueous NaHCO3 solution. The organic phase was dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated. The residue was purified by silica gel column chromatography (elution gradient: 0-100% EtOAc/hexane) to afford tert-butyl 3-cyano-3-((trimethylmethylsilyl)oxy)pyrrolidine-1-carboxylate (0.5 g, 1.758 mmol, 32.6% yield) and tert-butyl 3-cyano-3-hydroxypyrrolidine-1-carboxylate (0.306 g, 1.442 mmol. 26.7% yield).LC/MS analysis of tert-butyl 3-cyano-3-((trimethylmethylsilyl)oxy)pyrrolidine-1-carboxylate (Condition N-1): [M+H]+ 213.2, retention time (RT) = 4.359 min.1H NMR (400 MHz, chloroform-d) δ ppm: 3.83-3.72 (m, 1H), 3.72- 3.43 (m, 3H), 2.33 (q, J=6.8Hz, 2H), 1.52-1.42 (m, 9H), 0.19-0.10 (m, 9H).