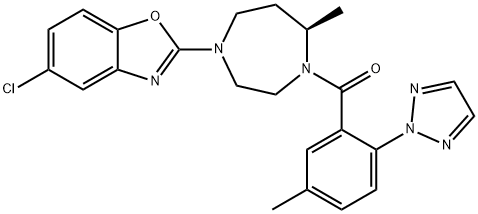
Commercially available acid 240 was first
subjected to a copper-assisted substitution reaction involving
1,2,3-triazole in DMF at elevated temperatures. Although these
conditions resulted in an excellent yield of a triazole-substituted
product, an approximate 4:1 ratio of the desired 2-arylated triazole
241 and the undesired 1-arylated triazole byproduct were recovered
from the reaction. The mixture was then treated with N,Ndimethylethylenediamine
in acid to sequester copper. Next, the
mixture of arylated triazoles was carefully subjected to sodium tbutoxide
in DMF and ethyl acetate to form the corresponding
sodium salts, and interestingly it was found that the desired
sodium salt of 241 could be isolated based on its solubility profile
under these conditions. Acidification of the desired carboxylate salt
using dilute HCl gave rise to acid 241 in 60% yield across the fourstep
sequence. Next, subjection of this acid to oxalyl chloride in
chilled DMF generated the acid chloride 242 in excellent yield. This
crude acid chloride was used immediately in the next step of the
synthetic sequence.
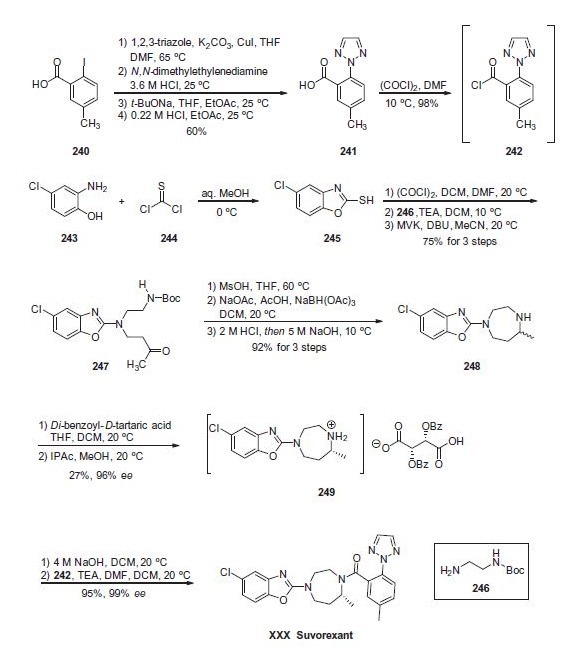
For the preparation of the diazepine-containing portion of
suvorexant, the synthesis commenced with the condensation of
commercial 2-amino-4-chlorophenol (243) with thiophosgene
(244) to furnish benzoxazole 245. Next, thiol 245
was converted to the corresponding chloride prior to exposure to
Boc-protected ethylenediamine 246 under basic conditions. This
was followed by a Michael addition of the resulting aminobenzoxazole
and methyl vinyl ketone (MVK). The result of this sequence of
reactions delivered aminobenzoxazole ketone 247 in 75% yield
over the three steps. Next, subjection of the carbamate to methanesulfonic acid removed the Boc functionality and this was
followed by an intramolecular reductive amination sequence to
construct the diazaepine ring. Acid¨Cbase workup ultimately provided
the racemic diazepine 248 in 92% yield from 247. Next, salt
formation with a benzoyl tartaric acid and subsequent recrystallization
upgrade using isopropyl acetate and methanol at ambient
temperature was used to resolve racemic 248 into the tartrate salt
249 in 27% yield and excellent enantiomeric excess. Finally, salt
249 was freebased using sodium hydroxide prior to exposure to
the crude acid chloride 242 under basic conditions to ultimately
deliver suvorexant (XXX) in 95% yield and 99% ee across the twostep
sequence.