What is Heptaldehyde?
Feb 12,2020
Heptanal or heptanaldehyde is an alkyl aldehyde. It is a colourless liquid with a strong fruity odor, which is used as precursor to components in perfumes and lubricants.
Emde et al. reported [1] its application on the total syntheses of two acetogenins, squamocin A and squamocin D, which exhibted selective cytotoxicity of on T24 bladder cancer cells at the S-phase via a Bax-, Bad-, and caspase-3-related pathways. The adjacent bis-THF subunit was constructed by a multiple Williamson reaction. The left and the right side chain were added by addition of organomagnesium compounds to aldehyde functions. The conversion of a carboxylic acid into the butenolide moiety concluded both syntheses.
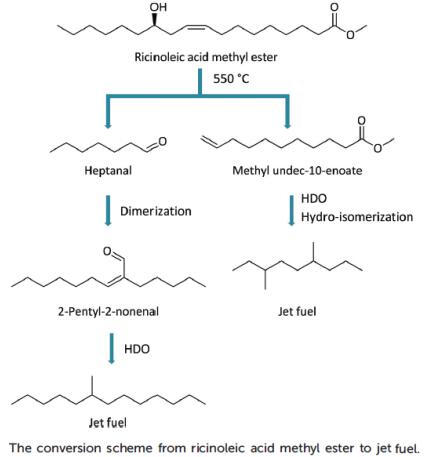
Zhou et al. reported [2] its application on selective conversion of castor oil derived ricinoleic acid methyl ester into jet fuel. A unique hydroxyl group in the ricinoleic acid chain induced a special thermal rearrangement reaction in medium chain fatty acid methyl ester (FAME) and heptanal formation. Methyl 10-undecenoate was converted into a branched paraffin with limited carbon loss. Both Pt/Al2O3 and Pt/ZSM-22 catalysts resulted in selective hydrogenation (with limited decarbonylation) and hydroisomerization (with negligible cracking), respectively. Another product, heptanal, was also converted into jet fuel through selective dimerization and hydrodeoxygenation with a supported amine and commercially available Pt/Al2O3 catalyst, respectively. With the right integration of these technologies, the carbon selectivity in the castor oil to jet fuel process is as high as 90%.

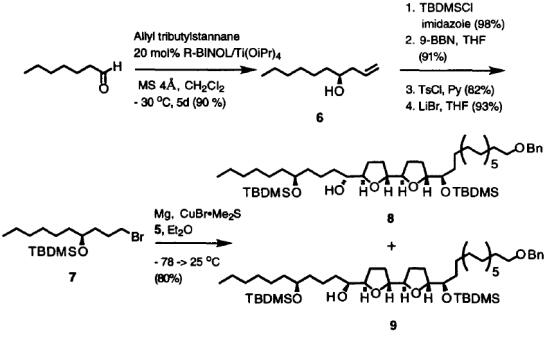
Yu et al. reported [3] its application on asymmetric allylation. An efficient method for the catalytic enantioselective addition of allyltributylstannane to aldehyde is found which employs a molecular accelerator, R2MSRA, and 1–2 mol% of BINOL–TiIV, furnishing homoallylic alcohol in good yield with useful levels of enantioselectivity. This approach to increasing catalytic ability by the use of synergetic reagents could be widely applicable, not only in the field of asymmetric carbonyl addition, but also in other related reactions.
References
1.Emde U, Koert U. et al. Total syntheses of squamocin A and squamocin D[J]. Tetrahedron Letters, 1999, 40(33): 5979-5982
2.Zhou YP. et al. Selective conversion of castor oil derived ricinoleic acid methyl ester into jet fuel[J]. Green Chem., 2016, 18:5180–5189
Yu CM. et al. Bifunctional molecular accelerator for catalytic asymmetric allylation: R2MSRA (M = B, Al) as a useful synergetic reagent[J]. Chem. Commun., 1997, 761-762
);
You may like
What is terpineol used for?
Feb 26, 2024
Lastest Price from Heptaldehyde manufacturers
Heptaldehyde

US $0.00/KG2023-06-30
- CAS:
- 111-71-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month
Heptaldehyde

US $0.00-0.00/KG2023-01-10
- CAS:
- 111-71-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt