The biosynthesis of rutin
Introduction
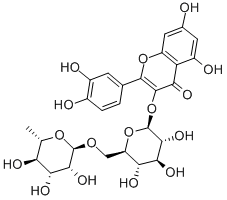
Rutin is widely found in nature and is almost contained in all of the Rutaceae and Sectaceae plants. As a flavonoid substance, rutin has a significant protective effect on the cardiovascular system. Rutin and its derivatives have a strong free radical scavenging effect, of which rutin has the strongest antioxidant activity. Clinically, rutin is mainly used for the adjuvant treatment of hypertension and treatment for the prevention of other bleedings due to lack of rutin, such as cerebral hemorrhage. Structural modification of rutin can generate a series of derivatives with good activities, such as troxerutin, which is well water-soluble and conducive to human absorption. Troxerutin has a more significant efficacy than rutin and is used in the treatment of varicose veins/venous disorders. Since rutin has a mild effect with low cost and less adverse reactions, especially its remarkable effect on acute cerebral infarction, it is of great worth on the promotion and application of rutin[1].

Picture 1 Rutin powders
Rutin biosynthesis
Flavonoids are distributed widely in plants and take part in many plant biological activities. Flavonoid metabolism is both highly conserved in many plant species[2]. Flavonoid biosynthesis in higher plants has been well defined and several key enzymes of the various flavonoid pathways have been identified. The main steps of the flavonoid pathway start with phenylpropanoid metabolism. Phenylpropanoid is converted to nine types of chemicals, including isoflavonoids, anthocyanins, flavones, and flavonols. Several major components of this pathway, such as 4-coumaroyl CoA ligase (4CL) gene, have been identified (Ehlting et al. 1999). Key functional genes, including 4CL, chalcone synthase (CHS) and dihydroflavonol 4-reductase (DFR) have been isolated from several plants species, such as zea mays L., petunia, P. tomentosa and A. thaliana. However, none of these genes have been reported in F. tartaricum until now. Previous studies on biosynthesis and the accumulation of rutin have mainly focused on key enzymes, such as rutin-degrading enzyme (RDE), converting rutin to quercetin, the upstream product of rutin biosynthesis. The existence of RDE in F. tartaricum seeds reduces the extractability and usability of the rutin present in the plant tissue.
Quercetin and Rutin
Quercetin and Rutin are most common flavone constituents of some herb extracts such as Hippophae rhamnoides L. Inter and intra herb pharmacokinetics interactions of Quercetin and Rutin were investigated in the present study. Pharmacokinetic study was investigated in the two groups of rats (n = 6) for pharmacokinetic interactions between the Quercetin and Rutin (2.5 mg/kg) mixture treated alone with European patented polyherbal formulation containing equivalent weight of the above[3]. The total plasma concentrations of Quercetin and Rutin were determined by liquid chromatography mass spectrometry (LC–MS). A method was developed and validated according to the ICH guidelines. The results of the present study shows that there are great differences in the pharmacokinetics of Quercetin and Rutin when they are administered together and from the polyherbal formulation which will be interacted by many other constituents. The bioavailability of Quercetin was lowered from the polyherbal formulation when compared with the co-administration, whereas the Rutin bioavailability has increased from the polyherbal formulation when compared with the co-administration. The maximum plasma concentration of Quercetin from coadministration and polyherbal formulation was 165.3 ± 31.9 and 90.8 ± 21.4 ng/mL, respectively, whereas in the case of Rutin it was 61.1 ± 29.3 and 121.7 ± 19.2 ng/mL. After polyherbal formulation administration to rats the AUC0–24, AUC0–∞ and AUMC0–∞ of both Quercetin and Rutin significantly increased when compared to co-administration. The above results proved that inter and intra herb pharmacokinetic interactions between Quercetin and Rutin. Possible interactions of the other constituents with hydrolyzing enzymes in the formulation enhances the oral bioavailability of Rutin. Accordingly besides the drug herb interactions, inter and intra herb interaction might be brought into view with the wide use of herbal remedies.
Reference
1 Natural Small Molecule Drugs from Plants, 2018
2 Sun, Z., Hou, S., Yang, W. et al. Exogenous application of salicylic acid enhanced the rutin accumulation and influenced the expression patterns of rutin biosynthesis related genes in Fagopyrum tartaricum Gaertn leaves. Plant Growth Regul 68, 9–15 (2012).
3 Kammalla, A.K., Ramasamy, M.K., Chintala, J. et al. Comparative pharmacokinetic interactions of Quercetin and Rutin in rats after oral administration of European patented formulation containing Hipphophae rhamnoides and Co-administration of Quercetin and Rutin. Eur J Drug Metab Pharmacokinet 40, 277–284 (2015).
);You may like
Related articles And Qustion
See also
Lastest Price from Rutin manufacturers

US $0.00/KG2024-04-28
- CAS:
- 153-18-4
- Min. Order:
- 1KG
- Purity:
- ≥98% HPLC
- Supply Ability:
- 1000KG

US $0.00/kg2024-04-27
- CAS:
- 153-18-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000 kg