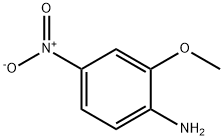
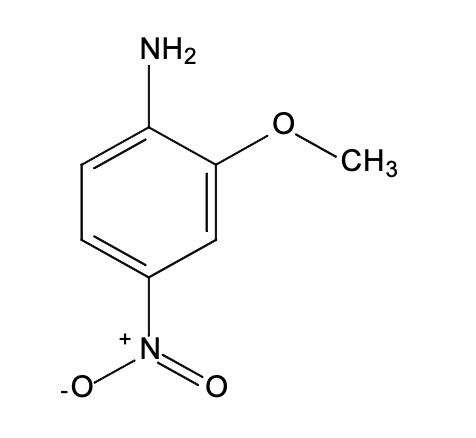
Synthesis and Toxicity of 2-Methoxy-4-nitroaniline
General description
2-Methoxy-4-nitroaniline is a chemical compound with significant industrial applications, including its use in dye manufacturing and as an intermediate in the synthesis of other compounds. 2-Methoxy-4-nitroaniline is characterized by the presence of a methoxy group and a nitro group attached to an aniline ring. This configuration contributes to its chemical behavior and reactivity, which are crucial in understanding its interaction with biological systems and the resulting toxicity. Its stability under certain conditions also influences how it should be handled and stored to minimize exposure risks[1].
Synthesis
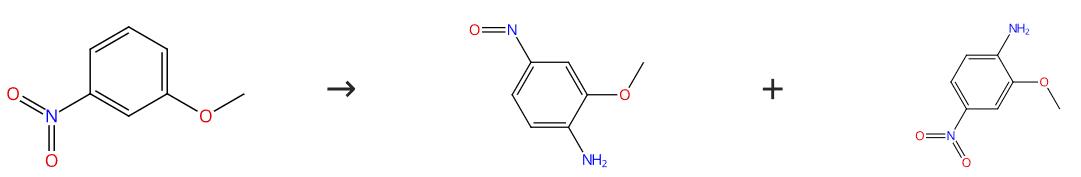
Fig. 1 The synthesis route of 2-Methoxy-4-nitroaniline
2.0 g (34 mmol) of acetamide, 4.1 g (68 mmol) of potassium hydroxide, 10 g of anhydrous potassium carbonate, 200 mg of pyrene, and 15 ml of DMSO were added to a 100 ml reactor equipped with a condenser and a thermometer, and the mixture was heated with stirring in the oxygen atmosphere. As the reaction temperature reached 85°C, 1.7 g (11 mmol) of 3-nitro anisole was added dropwise to the mixture through a dropping funnel for about 10 minutes. Extreme caution was used lest the temperature in the reactor should exceed 85±4°C. After 2 hours of the reaction, post-treatment was performed as described in Example 1. According to quantitative analysis, the conversion rate of 3-nitro anisole was 100% and the yield of the individual product based on 3-nitro anisole was 29 mole % for 2-methoxy-4-nitrosoaniline and 14 mol % for 2-methoxy-4-nitroaniline (the HPLC correction value of 2-methoxy-4-nitrosoaniline was that of 4-nitroguanidine). 2-methoxy-4-nitrosoaniline, yield 29 mole %; 2-methoxy-4-nitroaniline, yield 14 mol %. The synthesis route is shown in Fig. 1.
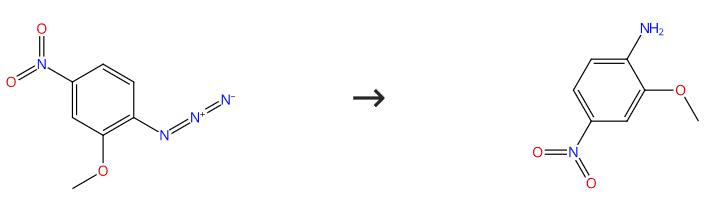
Fig. 2 The synthesis route of 2-Methoxy-4-nitroaniline
Add the thiazolium salt (3.30 mg, 0.0122 mmol) to a solution of 1-azido-4-nitrobenzene (0.122 mmol) and t-BuOH (18.0 mg, 0.244 mmol) in degassed THF (0.50 mL) under argon at room temperature. Stir the resulting suspension for 5 min and add NaOt-Bu (23.4 mg, 0.244 mmol) in one portion. Stir the mixture for 12 hours and add water (2 mL). Extract with EtOAc (2 × 2 mL) and dry the combined organic phase over and MgSO4. Filter and concentrate in vacuo. Purify by passing the resulting residue through a short pad of silica (elute with 50% light PE-EtOAc)[2].
Mechanism of Toxicity
The toxicity of 2-Methoxy-4-nitroaniline is primarily attributed to its metabolism within biological systems, leading to the formation of reactive intermediates. These intermediates can cause oxidative stress, resulting in cellular damage. Specifically, the nitro group undergoes enzymatic reduction, generating nitrosamines, which are known to be cytotoxic and potentially carcinogenic. Furthermore, the interaction with cellular macromolecules like DNA and proteins disrupts normal cellular functions, leading to toxicological consequences.
Evidence of Toxicity
Research studies and toxicological assessments have provided evidence of 2-Methoxy-4-nitroaniline's toxic effects. Acute exposure has been linked to methemoglobinemia, a condition where an abnormal amount of methemoglobin is produced, impairing the blood's ability to carry oxygen. Chronic exposure, primarily in industrial settings, has raised concerns about its potential carcinogenicity, though conclusive long.
Regulatory Guidelines and Safety Measures
Given its potential toxicity, 2-Methoxy-4-nitroaniline is subject to regulatory scrutiny. Various international agencies have set guidelines to limit exposure and ensure safe handling. In workplaces, the use of personal protective equipment (PPE), such as gloves and respirators, is recommended to minimize inhalation and skin contact. Environmental controls are also advised to prevent its release into the environment.
Conclusion
2-Methoxy-4-nitroaniline's utility in industrial applications cannot overshadow the importance of understanding and managing its toxicological risks. The compound's potential to cause significant health issues necessitates strict adherence to safety protocols and regulatory guidelines. Ongoing research and regulatory evaluations are essential to fully elucidate its toxicity profile and ensure that its use remains within safe boundaries. By prioritizing safety and health, we can mitigate the risks associated with 2-methoxy-4-nitroaniline and similar compounds.
References
[1]Degawa M, Nakayama M, Konno Y, et al. 2-Methoxy-4-nitroaniline and its isomers induce cytochrome P4501A (CYP1A) enzymes with different selectivities in the rat liver[J]. Biochimica et Biophysica Acta (BBA)-General Subjects, 1998, 1379(3): 391-398.
[2]Burnley, James; et al.Catalytic reduction of ortho- and para-azidonitrobenzenes via tert-butoxide ion-mediated electron transfer Synlett (2013), 24(5), 652-656.
);You may like
Related articles And Qustion
Lastest Price from 2-Methoxy-4-nitroaniline manufacturers

US $100.00/KG2023-08-26
- CAS:
- 97-52-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5000KG

US $10.00/PCS2023-01-10
- CAS:
- 97-52-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt