Olivetol: Natural Occurrence, Biotransformation and Electrochemical Detection
General Description
Olivetol, a naturally occurring compound derived from plants in tropical regions, offers various health benefits due to its antioxidant, anti-inflammatory, and antimicrobial properties. Biotransformation by Syncephalastrum racemosum yields three metabolites, providing insights into its metabolic pathways. Electrochemical detection using a p-L-serine/CuO/CPE sensor enhances olivetol analysis, crucial for cannabinoid synthesis research. This sensor offers sensitive and quantitative detection with a wide linear range and low detection limit. Overall, olivetol's natural origin, metabolic pathways, and efficient detection methods contribute to its significance in health, biotechnology, and environmental applications.

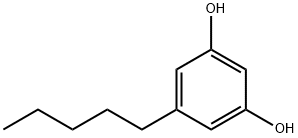
Figure 1. Olivetol
Natural Occurrence
Olivetol, a naturally occurring compound, is derived from various plants, predominantly found in tropical and subtropical regions. This natural origin ensures its purity and biocompatibility, making it highly desirable for a multitude of applications. Extracted through meticulous processes, Olivetol retains the beneficial properties of the plants it originates from, providing a range of health benefits. Its unique chemical structure grants it antioxidant, anti-inflammatory, and antimicrobial properties, making it a valuable addition to many personal care and healthcare products. The plants from which Olivetol is derived are rich sources of bioactive compounds, which contribute to its therapeutic properties. These plants often thrive in nutrient-rich environments, absorbing minerals and phytonutrients from the soil, which are then concentrated in their extracts. This ensures that Olivetol maintains its potency and efficacy. Furthermore, Olivetol's natural origin promotes sustainability, as it can be sourced from renewable plant resources. This not only reduces dependence on synthetic alternatives but also supports environmentally friendly practices. By utilizing plant-derived Olivetol, companies can align with the growing demand for eco-friendly and sustainable products, meeting consumer preferences for natural ingredients. In summary, Olivetol's natural origin guarantees its safety, effectiveness, and biocompatibility while also contributing to environmental sustainability, making it a preferred choice for a wide range of applications. 1
Biotransformation
Biotransformation of olivetol by Syncephalastrum racemosum ATCC 18192 results in the production of three metabolites: 4'-hydroxy-olivetol, 3-(3,5-dihydroxyphenyl)-1-propanol, and 3-(3,5-dihydroxyphenyl)-1-propanoic acid. These metabolites were identified through spectral analysis (pmr, cmr, ms) and comparison with olivetol. The absolute configuration of 4'-hydroxy-olivetol was determined to be R using the Horeau partial resolution method. This study suggests that the biotransformation process involves a subterminal oxidation mechanism. This research sheds light on the metabolic pathways of olivetol in Syncephalastrum racemosum, providing valuable insights into the biochemical transformations of this compound. Understanding such biotransformation processes is crucial for various applications, including biotechnology, pharmaceuticals, and environmental remediation. Further studies may explore the enzymatic mechanisms involved and optimize the biotransformation process for industrial and scientific purposes. 2
Electrochemical Detection
The electrochemical detection of olivetol using a p-L-serine/CuO/CPE (Poly(L-Serine) Film Layered Copper Oxide Modified Carbon Paste Electrode) represents a significant advancement in sensor technology for analyzing this important polyphenol compound, particularly relevant in cannabinoid synthesis due to its diverse biological activities. This innovative sensor design integrates p-L-serine and copper oxide (CuO) nanoparticles onto a carbon paste electrode (CPE), enhancing the electrode's performance for olivetol detection. The sensor's efficacy was evaluated through cyclic voltammetry (CV) and differential pulse voltammetric (DPV) techniques. Compared to bare CPE and CuO/CPE, the p-L-serine/CuO/CPE demonstrated superior electrochemical signal enhancement for olivetol. Under optimized conditions, this sensor exhibited a wide linear analysis range (20 to 100 μmol L-1) with a low detection limit (1.04 μmol L-1), ensuring sensitive and quantitative olivetol determination. The fabrication process leverages cost-effective and readily available materials, making the p-L-serine/CuO/CPE an attractive choice for olivetol detection. The sensor's reproducibility, repeatability, and stability further validate its practical utility. Overall, this electrochemical sensor presents a novel, efficient, and economical approach for olivetol analysis, highlighting its potential as a valuable tool in cannabinoid synthesis research and related biological studies. 3
Reference
1. Olivetol. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 10377.
2. McClanahan RH, Robertson LW. Biotransformation of olivetol by Syncephalastrum racemosum. J Nat Prod. 1984; 47(5): 828-834.
3. You Z, Zhang Y, Duan S, Liu L. Electrochemical Detection of Olivetol Based on Poly(L-Serine) Film Layered Copper Oxide Modified Carbon Paste Electrode (p-L-Serine/CuO/CPE). Nanomaterials (Basel). 2022; 13(1): 70.
Related articles And Qustion
See also
Lastest Price from Olivetol manufacturers

US $0.00/kg2025-04-30
- CAS:
- 500-66-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $1.00/g2025-04-21
- CAS:
- 500-66-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg