Synthesis and Detection method of Bisoprolol fumarate
General description
Bisoprolol fumarate is a selective beta1-adrenergic receptor blocker. No intrinsic sympathomimetic activity and membrane stabilization. Different model animal experiments show that its affinity with β1-receptor is 11 to 34 times greater than that of β2-receptor, and its selectivity for β1 receptor is 4 times that of the similar drug Atenolol. This product has a long duration of action (more than 24 hours), and continuous use can control symptoms well without tolerance. It has minimal side effects on the respiratory system and has no effect on fat catabolism. There is also a certain degree of blockade of bronchial β2 receptors, but it may only appear in large doses, and generally has no obvious clinical significance.
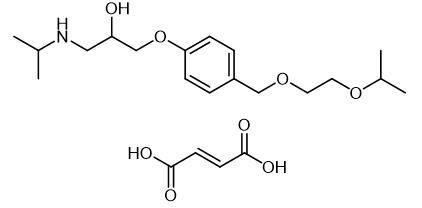
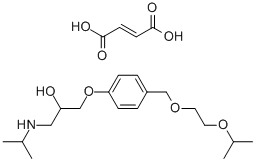
Fig. 1 The structure of Bisoprolol fumarate.
Synthetic routes
Step 1
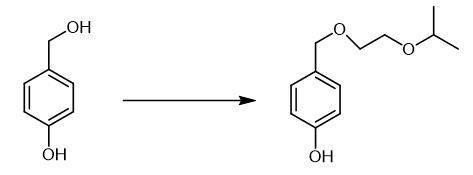
Fig. 2 The synthetic step 1 of Bisoprolol fumarate.
In a 250 L reactor 8.33 kg of sodium hydroxide was dissolved in water and 25.0 kg of 4-Hydroxy benzaldehyde was added. The reaction mixture was stirred to obtain a clear solution. The reaction mixture was cooled to 15 °C and added a solution of 3.0 kg Sodium borohydride dissolved in 15 L of water at 15 - 20 °C in about 3 hrs. The reaction mixture was further stirred for 3 hrs. 1.0 kg Charcoal slurry prepared in 15 L water was added to reaction mixture and stirred further for 30 minutes and filtered over celite bed. The reaction mixture was collected in 400 L reactor and chilled to 0 - 5 °C. Dilute solution of acetic acid (20 kg diluted with 20 L water) was slowly added to the reaction mixture at 0 -5 °C in a period of 3 - 5 hrs to get the product separated. The separated product was filtered and washed with 40 L water, spin dried and dried under vacuum to obtain 21 - 23 kg 4-Hydroxy benzyl alcohol. 4-Hydroxy benzyl alcohol, Yield 21 - 23 kg [1].
Step 2
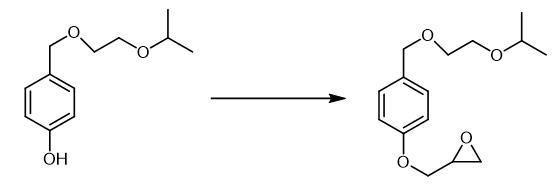
Fig. 3 The synthetic step 2 of Bisoprolol fumarate.
In a 400 L reactor, 280 L of 2-Isopropoxy ethanol was charged and cooled to 0 °C. Amberlyst-15 (22.5 kg) resin was added to it in one lot. 4-Hydroxy benzyl alcohol (22.5 kg) was added to it in small lots of 2 kg each at 0 -5 °C in about 5 hrs. The reaction mixture yas stirred at 0 - 5 °C for 2 hrs. The temperature was raised to 15 - 20°C and maintained for 10 hrs. The Amberlyst-15 resin was filtered and washed with 2-Isopropoxy ethanol. The reaction mixture was collected in a 400 L vessel and basified with 1.0 kg of potassium carbonate. The potassium carbonate was filtered and the reaction mixture was taken for distillation of 2-Isopropoxy ethanol to get 36 - 38 kg of 4-[(2-isopropoxyethoxy)methyl]-phenol. 4-[(2-isopropoxyethoxy)methyl]-phenol, Yield 36 - 38 kg [1].
Step 3
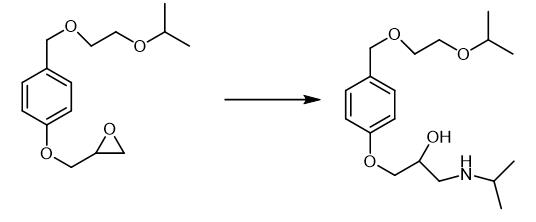
Fig. 3 The synthetic step 3 of Bisoprolol fumarate.
The aqueous solution of sodium salt of 4-[(2-Isopropoxyethoxy)methyl]-phenol was reacted with 90 kg of Epichlorohydrin at 60-65 °C for 1 hr. The reaction mixture was then extracted twice with 90 L toluene. The toluene extracts was stirred with 7.2 kg solid Sodium hydroxide. The reaction mixture was washed with water three times and the toluene layer was taken for distillation. 2-[[4-(2-isopropoxyethoxy)-methyl]-phenoxymethyl]oxirane was obtained as an oil after the removal of solvent. The product was further purified by high vacuum distillation at 0.5 mm at 160-200 °C to get purified 2-[[4-(2-isopropoxyethoxy)-methyl]phenoxymethyl]oxirane. 2-[[4-(2-isopropoxyethoxy)-methyl]phenoxymethyl]oxirane [1].
Step 4
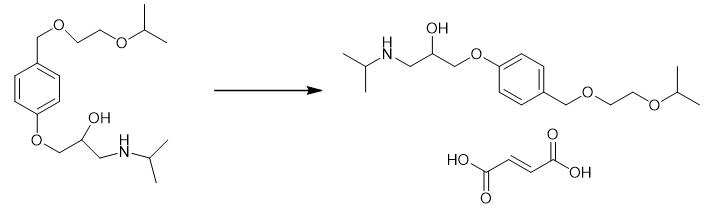
Fig. 4 The synthetic step 4 of Bisoprolol fumarate.
In a 160 L vessel, 60 L methanol was charged and 30 kg of 2-[[4-(2-isopropoxyethoxy)-methyl]-phenoxymethyl]oxirane was added. The reaction mixture was cooled to 15 °C and 0.3 kg of Sodium borohydride was added in small lots of 30 gm each to the reaction mixture at 15 - 20 °C. The reaction mixture was stirred for 1 hr at 15 - 20 °C and was added to a cooled solution of isopropyl amine at 15 - 20 °C in about 1hr. The reaction mixture was stirred for 3 hrs and heated to reflux for 3hrs. The excess of Isopropyl amine and methanol was distilled out of the reaction mixture. The residual oil was taken in 60 l chloroform and washed three times with water. The chloroform layer was then passed over a bed of 30 kg neutral allumina in about 4-6 hrs and washed with another 30 L chloroform. Chloroform was distilled out and degassed to obtain 34 - 37 kg bisoprolol base as oil. Preparation of bisoprolol fumarate 100 l of Acetone and 36 kg of bisoprolol base were taken in a 160 L vessel. The reaction mixture was heated to 40 °C and 6.54 kg fumaric acid was added. The reaction mixture was stirred at reflux for 30 minutes, cooled to 0 - 5 °C and maintained for 1 hr. The separated product was centrifuged and washed with 4x10 L chilled acetone. The product was spin dried for 15 minutes to get 30 - 33 kg of bisoprolol fumarate. bisoprolol fumarate, Yield 30 - 33 kg [1].
Detection method
Two simple, precise and economical spectrophotometric methods have been developed for the estimation of Bisoprolol Fumarate in bulk and pharmaceutical formulations. Bisoprolol Fumarate shows a sharp peak at 220.0 nm in first order derivative spectrum with n = 1 ( Method A). Method B applied is based on calculation of Area under Curve (AUC) for analysis of Bisoprolol Fumarate in the wavelength range of 218-228 nm. The drug follows the Beer-Lambert's law in the concentration range of 5-50 mu g/ml in both the methods. Results of the analysis were validated statistically and by recovery studies and were found to be satisfactory [2].
For the simultaneous quantification of Ivabradine HCl and Bisoprolol fumarate in bulk and synthetic mixtures, a sensitive, precise, robust, and accurate reversephase-high-performance liquid chromatographic technique was developed and validated according to the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Q2 (R1) guideline. The Phenomenex C-18 column (250 x 4.6 mm(2), 5 m) was equilibrated with mobile phase acetonitrile: KH2PO4 (30:70, v/v), pH 5.0 was adjusted with 1% orthophosphoric acid, the flow rate was maintained at 1 ml/min, and analysis was performed using a photodiode array detector. Note that, 279 nm was found to be the common detection wavelength for Ivabradine HCl and Bisoprolol fumarate. As per the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use criteria, the technique was validated for linearity, precision, accuracy, and robustness, as well as the limit of detection and limit of quantitation. The linearity range of Ivabradine HCl and Bisoprolol fumarate was found to he 5-40 and 2.5-20 mu g/ml and correlation coefficients were 0.9988 and 0.9994, respectively. The mean percentage recovery of Ivabradine HCl and Bisoprolol fumarate wa);
See also
Lastest Price from Bisoprolol fumarate manufacturers

US $100.00/kg2024-05-08
- CAS:
- 104344-23-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10

US $0.00/KG2024-03-16
- CAS:
- 104344-23-2
- Min. Order:
- 100g
- Purity:
- 98%+
- Supply Ability:
- 100kg