Synthesis and Application of Toltrazuril
General description
Toltrazuril is a triazinone compound, which is a novel broad-spectrum special anticoccidial drug. Toltrazuril is a white or off-white crystalline powder; odorless. Soluble in ethyl acetate or dichloromethane, slightly soluble in methanol, insoluble in water.
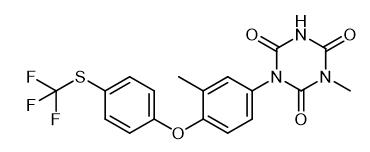
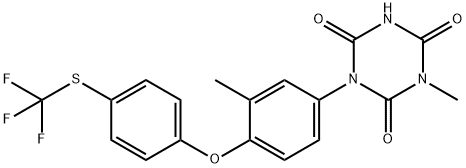
Fig. 1 The structure of Toltrazuril.
Synthetic routes
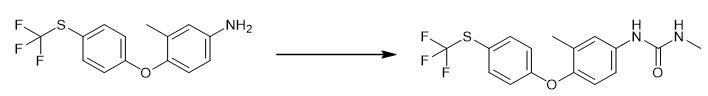
Fig. 2 The synthetic step 1 of Toltrazuril.
In a 500 mL four-neck round bottom flask, add 29.9 g (0.1 mmol) of 3-methyl-4-(4-trifluoromethylthio-phenoxy)-aniline, 180 mL of toluene, 16.8 g of methylcarbamoyl chloride ( 1.8 eq), reacted at 60°C for 3 h, TLC monitored and tracked the reaction, when the reaction of the raw materials was complete, the temperature was first cooled to room temperature, and 1M NaOH was slowly added dropwise to adjust to pH=7, the system became turbid, and then the temperature was raised to make the system clarified. The layers were separated, the organic layer was taken, the aqueous layer was extracted with toluene (50 mL×2), the toluene layer was taken, the toluene layers were combined, and the toluene was evaporated under reduced pressure to obtain pale yellow 1-methyl-3-[3-methyl- 4-(4-Trifluoromethylthio-phenoxy)-phenyl]-urea crude solid 34.1 g, molar yield of 90%, for use [1].
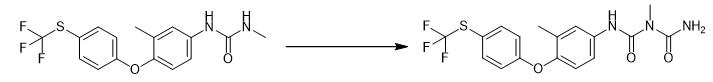
Fig. 3 The synthetic step 2 of Toltrazuril.
The crude 1-methyl-3-[3-methyl-4-(4-trifluoromethylthio-phenoxy)-phenyl]-urea obtained above was dissolved in 30 mL of acetone, at room temperature , stir for 30 min, add acetic acid 18 g (3 eq) dropwise, the dropwise addition time is 60 min, continue to stir for 20 min, then add dropwise 88 mL of sodium cyanate aqueous solution (concentration is 110 g/L), add dropwise for 1.5 h, the drop is completed, and the temperature is raised to 40°C, along with the carrying out of reaction, milky white solids are successively separated out in the system, continue insulation reaction 8 h, be cooled to 10℃, add 100 mL water, make solid separate out completely, filter, oven dry, get off-white 3-methyl-1 -[3-methyl-4-(4-trifluoromethylthiophenoxy)phenyl]-biuret solid 34.3 g, molar yield 95%[1].

Fig. 3 The synthetic step 3 of Toltrazuril.
In a 500 mL round-bottomed flask, add 60 g diethyl carbonate (5 eq), 12.0 g sodium hydride (3 eq, sodium hydride is stored in paraffin, the content is about 60%), stir and mix, and heat up to 60°C, dropwise Add 34.8 g of 3-methyl-1-[3-methyl-4-(4-trifluoromethylthiophenoxy)phenyl]-biuret prepared above, dissolve in 75 mL of toluene, and the dropwise addition time is 90 min, dripping was completed, the reaction was incubated for 3 h, cooled to room temperature, slowly added dropwise with acetic acid (30 mL), adjusted the pH of the system to 7, then slowly added water (90 mL), the system was pale yellow and turbid, warmed to 70 °C, the system was dissolved Clarify, stand for stratification, separate liquids, take the organic layer, and then extract the aqueous layer with toluene (50 mL×2), and take the organic layer, combine the organic layers, and evaporate the organic solvent under reduced pressure to obtain a pale yellow toltrazuril crude solid, And it was recrystallized with methanol to obtain 35.1 g of pure product, the molar yield was 95%, the content measured by HPLC was 99.6%, and the total yield was 81%. IR (KBr) νmax= 3328, 3126, 1665, 1687, 1611, 1486, 1468, 1309, 1288, 1264, 1225, 1213, 1165, 1118, 1011, 887, 845, 756 cm-1;MS (ESI) 424.1 [M-1]-; HRMS(ESI) calcd for C18H14F3N3O4SNa [M+Na]+448.0549;found 448.0558[1].
Detection Method
Ultra high performance liquid chromatography with UV detection was developed and validated for the simultaneous determination of residues of toltrazuril and its two metabolites, namely, toltrazuril sulphone and toltrazuril sulphoxide, in edible tissues (chicken and porcine muscle, liver and kidney). Acetonitrile was used to extract analytes from tissues, which were then cleaned using primary secondary amine and Oasis (TM) MAX solid phase extraction cartridges. Chromatographic separation was performed on a C-18 column with gradient elution. The analytes were determined using a UV detector. The regression coefficients of matrix-matched calibration curves showed linearity of > 0.99. Calibration ranged from 25 to 1,000 mu g kg-1 for toltrazuril and toltrazuril sulphone, and 37.5-1,500 mu g kg-1 for toltrazuril sulphoxide. The accuracy was between 80 and 110 %, and the inter and intraday RSDs were lower than 15.2 and 18.3 %, respectively. The limits of detection for toltrazuril, toltrazuril sulphone and toltrazuril sulphoxide were between 10 and 37.5 mu g kg-1 in all the tissues. The developed method was successfully applied to detect toltrazuril, toltrazuril sulphone and toltrazuril sulphoxide in tissues of medicated chicken [2].
Application
Anticoccidial
A 42-day broiler floor pen study was conducted comparing the anticoccidial efficacy of toltrazuril (Baycox) as a stand alone treatment and as an additional treatment to in-feed anticoccidial programs. Toltrazuril was administered on days 18 and 19 in the drinking water at 7 mg/kg of body weight. The treatments were 125 ppm nicarbazin (days 0-14) to 66 ppm salinomycin (SAL) (days 15-35) with and without toltrazuril, SAL (days 0-35) with and without toltrazuril, nonmedicated (NM) to SAL with toltrazuril, and NM with and without toltrazuril. The controls were NM noninfected and infected. The treatments were replicated in five blocks of eight pens each in a randomized complete block design. All withdrawal feed was nonmedicated. On day 14, birds, except noninfected, were exposed to coccidial oocysts (Eimeria acervulina, Eimeria maxima, and Eimeria tenella) seeded litter. On days 21, 28, 35, and 42, birds and feed were weighed, four birds per pen were coccidial lesion scored, and litter oocyst counts were performed. The coccidial infection in the NM infected treatment caused a significant (P < 0.05) coccidiosis infection. Coccidiosis was moderately controlled in the anticoccidial treatment birds without toltrazuril. Performance in the NM with toltrazuril was equal to or better (P < 0.05) than the anticoccidial programs without toltrazuril. Toltrazuril was equal to the noninfected birds in performance. Toltrazuril most completely eliminated all coccidial lesions and dramatically reduced oocyst shedding. The performance data, lesion scores, and oocyst counts showed that a 2-day treatment with toltrazuril successfully controlled the coccidiosis with no relapse of infection. Toltrazuril can thus be used for supplemental control with in-feed anticoccidials or as a primary anticoccidial with nonmedicated feed [3].
Neospora caninum infection
C57BL/6 mice were infected with Neospora caninum tachyzoites during pregnancy, yielding a transplacental infection of developing fetuses. Subsequently, congenitally infected newborn mice were treated either once or three times with toltrazuril (or placebo) at a concentration of 31.25 mg c);
You may like
See also
Lastest Price from Toltrazuril manufacturers

US $0.00/Kg/Bag2024-04-28
- CAS:
- 69004-03-1
- Min. Order:
- 2Kg/Bag
- Purity:
- 99% up, High Density
- Supply Ability:
- 20 tons

US $10.00/kg2024-04-25
- CAS:
- 69004-03-1
- Min. Order:
- 1kg
- Purity:
- 99.8%
- Supply Ability:
- 10000ton