Synthesis and Bioactivity of Mesterolone
General description
Mesterolone is a synthetic androgen and dihydrotestosterone derivative. Met is rarely used as a replacement therapy because of its weak androgen activity.
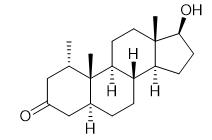
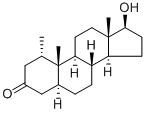
Fig. 1 The structure of Mesterolone.
Synthetic routes
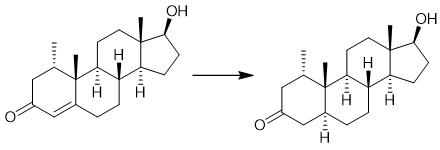
Fig. 2 The synthetic method 1 of Mesterolone.
In a mixture of 50 ml of THF, 19.0 ml of t-butanol and 40 ml of ammonia, 2.01 g (289.58 mmol) of lithium are dissolved at 45° C. The mixture is admixed at 50° C. with a solution of 32 g (105.79 mmol) of 1α-methyl testosterone in 210 ml of THF. After the reaction has ended, it is quenched by adding 10 ml of mesityl oxide. The reaction mixture is warmed to +20° C. and added to a suspension of 16 g of ammonium chloride and 50 ml of water. After phase separation the organic phase is added in 2 litres of ice water.The resulting suspension was adjusted to a pH of about 3 with HCl . Thereafter, the precipitated solid is isolated, washed to neutrality with water and dried at 50° C. Yield: 98-100% of weight of starting material [1].
Detection method
Mesterolone is a synthetic androgenic steroid indicating a weak anabolic activity. A new, simple in use, and economical TLC-densitometric method in normal phase system (NP-TLC) has been developed and validated for the identification and quantitative determination of mesterolone in bulk drug and in tablet formulation. NP-TLC analysis was performed on aluminium plates precoated with silica gel 60F(254) as the stationary phase using chloroform-acetone (40 : 10, v/v) as mobile phase. Densitometric analysis was carried out at lambda = 745nm after staining with phosphomolybdic acid. These conditions were found to give visible (dark blue) spot and sharp peak, respectively, for mesterolone at R-F 0.75 +/- 0.02 and enabled satisfactory separation of mesterolone from its related substance (potential impurity). The proposed NP-TLC-densitometric method was validated for specificity, linearity, precision, accuracy, robustness, and sensitivity according to ICH guideline and other validation requirements. The limit of detection (LOD) and limit of quantification (LOQ) were 61.0 ng.spot(-1) and 184.0 ng.spot(-1), respectively. The percent content of mesterolone in marketed tablet formulation was found to be 99.40% of label claim. The developed TLC-densitometric method can be successfully used in quality control of mesterolone in bulk material and also tablet formulation [2].
Bioactivity
Morphological changes in murine skeletal muscle
Light and electron microscopy and quantitative morphometry were used to determine the effects of exercise and mesterolone on the soleus muscles of mice. Both exercise and mesterolone caused a significant hypertrophy of extrafusal muscle fibres. The hypertrophy of Type I fibres was greater than that of Type II fibres. There was no hyperplasia. Mitochondria were more numerous and larger than in the muscles of sedentary animals. Capillarity increased and small centrally nucleated muscle fibres appeared, usually in small clusters and most often in the muscles of animals exposed to mesterolone. A small proportion of satellite cells exhibited signs of activation but there were more in the muscles of mesterolone-treated animals than after exercise. Muscles from animals that had been both exercised and treated with mesterolone exhibited the largest changes: muscle mass and muscle fibre hypertrophy was greater than in all other groups of animals, capillarity was higher and > 30% of all recognized satellite cells exhibited signs of activation. Groups of small centrally nucleated muscle fibres were commonly seen in these muscles. They appeared to be the result of splits in the form of sprouts from existing muscle fibres. With both exercise and mesterolone, alone or in combination, there was an increase in the proportion of Type I muscle fibres and a decrease in the proportion of Type II [3].
Influence on Satellite Cell Distribution and Fiber Morphology
Mesterolone is a synthetic oral anabolic androgenic steroid used to treat hypogonadism. There are frequent reports of mesterolone abuse in human and equine sports to increase muscle mass and strength. However, limited information is available about how this drug exerts its effects on skeletal muscle. Satellite cells (SCs) are mononuclear myogenic stem cells that contribute to postnatal muscle growth and repair. As SC activation and subsequent differentiation to new myonuclei is a major event during muscle hypertrophy, this study investigated the influence of mesterolone on SC distribution within the pectoralis muscle of chickens. Specifically, this study tested the hypotheses that mesterolone induces avian skeletal muscle hypertrophy, and that mesterolone increases the number of SCs in avian skeletal muscle. Robust immunocytochemical techniques and morphometric analyses were used to calculate the numbers of SCs and myonuclei. Also, DNA concentration and Pax7 protein levels were measured to confirm immunocytochemical findings. Mesterolone significantly increased pectoralis mass and fiber size. All SC indices and number of myonuclei increased significantly by mesterolone administration. In addition, greater DNA concentration and Pax7 protein expression were found in mesterolone-treated birds. This study indicates that mesterolone can induce avian skeletal muscle hypertrophy and that this is correlated with increased number of SCs. We suggest that SCs are key cellular intermediaries for mesterolone-induced muscle hypertrophy [4].
Effect on cardiac remodelling and lipoprotein profile
Abuse of anabolic-androgenic steroids (AAS) for improving physical performance is associated with serious, sometimes fatal, adverse effects. The aim of the present work was to investigate the effects of AAS on the cardiac structure and the plasma lipoprotein profile isolated and in combination with exercise. Transgenic mice with a human lipaemic phenotype (expressing cholesteryl ester transfer protein on the LDL receptor knockout background) were used in this study. Sedentary and exercised mice (treadmill running, five times per week for 6 weeks) were treated with mesterolone (2 mu g/g body weight) or vehicle (control-C) in the last 3 weeks. Four groups were compared: (i) exercise + mesterolone (Ex-M), (ii) exercise + vehicle (Ex-C), (iii) sedentary + mesterolone (Sed-M) and (iv) sedentary + vehicle (Sed-C). Arterial blood pressure and body mass increased in all groups along time, but Sed-M reached the highest values and Ex-C the lowest. Treatment with mesterolone increased total cholesterol, triglyceride, low-density lipoprotein cholesterol (LDL-c) and very LDL-c (VLDL-c) plasma levels. However, exercise blunted some of these deleterious effects by increasing high-density lipoprotein cholesterol and decreasing LDL-c, VLDL-c and triglycerides. Exercise training induced beneficial effects, such as physiological cardiomyocyte hypertrophy, increase in myocardial circulation and decrease in cardiac interstitium. However, mesterolone impaired such physiological gains and in addition increased troponin T plasma levels both in sedentary and exercised mice. Thus, while mesterolone induced pro-atherogenic lipoprotein profile and pathogenic cardiac hypertrophy, exercise counteracted these effects and modified favourably both the lipoprotein profile and the cardiac remodelling induced by mesterolone [5].
References
[1] Ortmann I, Haeselhoff C. Use of α,β-unsaturated carbonyl compounds as quenching reagents for Birch reduct);
You may like
Lastest Price from Mesterolone manufacturers

US $12.00-8.00/kg2024-04-30
- CAS:
- 1424-00-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg

US $1.00-68.00/g2024-04-30
- CAS:
- 1424-00-6
- Min. Order:
- 1g
- Purity:
- 99.99%
- Supply Ability:
- 1000kg