Synthesis and Application of 2, 3, 4, 5-Tetramethyl-2-Cyclopentenone
General description
2,3,4,5-Tetramethyl-2-Cyclopentenone is an important pharmaceutical intermediate, which can be used as a functional material intermediate. It can be prepared by the reaction of ketone with titanium trichloropropoxy and acetaldehyde.
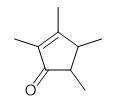
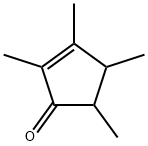
Fig. 1 The structure of 2,3,4,5-Tetramethyl-2-Cyclopentenone.
Synthetic routes
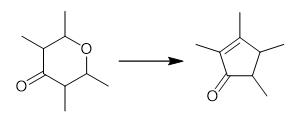
Fig. 2 The synthetic method 1 of 2,3,4,5-Tetramethyl-2-Cyclopentenone.
A 12-L three-necked round-bottom reaction flask was cooled in ice. It was charged with 6500 mL of 95-97% HCOOH and 2250 mL of H2SO4 and the solution allowed to cool to room temperature. The 2,3,5,6- tetrahydro-2,3,5,6-tetramethyl-7-pyrone, 1580 g (10.1 mol), was added with stirring to the acid solution at a fast trickle. The reaction solution was then heated to 50 °C and stirred for 16-24 h. The resulting dark brown organic mixture was next poured in 500-mL aliquots into 1000 g of ice. Ether (500 mL) was added and the aqueous phase was removed and back-extracted with 2 × 250 mL of ether. The combined ether layers were washed with 2 × 500 mL of 2 N NaCl and 10% NaOH until the aqueous layer became yellow. The aqueous layer was then checked with pH paper to ensure alkalinity. (Failure to remove all HCOOH from the organic layer results in product decomposition during the final distillation.) The organic layer was again washed with 2 N NaCl and the ether removed by rotary evaporation. The crude product was then purified by fractional distillation using a Vigreux column and water aspirator. The yellow liquid boiling at 77-90 °C (15 Torr) was collected. Yield: 1045 g (75%). 1H NMR (60 MHz, CCl4): 1.20 (m, 2), 1.30 (s, 3), 1.45 (s, 3), 1.80 (s, 3), 2.10 (s, 3 H) [1].
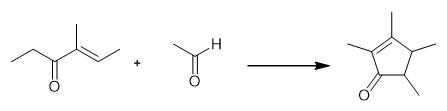
Fig. 3 The synthetic method 2 of 2,3,4,5-Tetramethyl-2-Cyclopentenone.
In a bottom round flask equipped with a mechanical stirrer, a dropping funnel and a reflux condenser was loaded 2000g (23.2 mol) of the starting ketone with 75% w/w of butylacetate as the solvent, 0.35 molar equivalents of anhydrous magnesium chloride and the aforementioned titanium catalytic solution containing 0.06 molar equivalents of the trichloropropoxytitanium complex. The resulting suspension was stirred vigorously and allowed to heat to 90°C. Then 2 molar equivalents of the acetaldehyde were added dropwise over 3h at 90°C. The reaction was continued for an additional hour and cooled to 40°C. The reaction mixture was hydrolysed with a 10% aqueous acetic acid solution and neutralised with a 20% aqueous potassium carbonate solution. The resulting organic phase was directly fractionated into a laboratory Sulzer packed column, to afford the title compound, as a mixture of isomers trans:cis = 85:15, in 27 % yield and the enone (II) (i.e. 4-methyl-4-hexen-3-one) in 31 % yield. mixture of isomers trans:cis = (B.p. = 70-80°C at P = 8 mbar); 4-methyl-4-hexen-3-one = (B.p. = 45-65°C at P = 8 mbar). 1H-NMR (isomer trans): 1.15 (d 3H); 1.19 (d 3H); 1.68 (s 3H); 1.88 (m 1H); 1.98 (s 3H); 2.25 (m 1H). 13C-NMR (isomer trans): 8.5; 14.6; 15.1 ; 17.7; 46.2; 48.4; 134.5; 171.6; 211.0 [2].
Application
For the synthesis of novel phenolate "constrained geometry" catalyst system
Chen et al. reports the convenient "one-pot" synthesis of a bifunctional -Me4Cp over arc phenolate(-) ligand and the efficient "one-step" synthesis of the corresponding Ti-IV and Zr-IV complexes. The reaction of 2-bromo-4-methylphenol with 2 equiv of (BuLi)-Bu-n followed by addition of 2,3,4,5-tetramethyl-2-cyclopentenone produces the bifunctional mono-Cp phenol ligand precursor 2-(tetramethylcyclopentadienyl)-4-methylphenol ((TCP)H-2, 1). Reaction of 1 with Ti(CH2Ph)(4) at 60 degrees C in toluene cleanly generates (TCP)Ti(CH2Ph)(2) (2), while the corresponding reaction with Zr(CH2Ph)(4) at higher temperatures affords the chelated C-2-symmetric zirconocene (TCP)(2)Zr (3). In solution at room temperature, the two benzyl groups of 2 are magnetically equivalent, however, in the solid state, X-ray diffraction reveals that one benzyl group is coordinated in a normal eta(1)-fashion and the other in an eta(2)-mode. The small Cp(centroid)-Ti-O angle of 107.7(2)degrees in 2 indicates sterically open features in common with amido-based "constrained geometry" polymerization catalysts. Low-temperature NMR-scale reactions of 2 with B(C6F5)(3) and Ph3C+B(C6F5)(4)(-) indicate the formation of the corresponding cationic complexes (TCP)TiCH2Ph+ PhCH2B(C6F5)(3)(-) (4) and (TCP)TiCH2Ph+B(C6F5)(4)(-) (5). Upon activation with Ph3C+B(C6F5)(4)(-), complex 2 is highly active for ethylene, propylene, and styrene polymerization [3].
For the synthesis of (R)-cyclopentadienyl-binaphthoxy titanium(IV) complexes
Two new chiral pre-ligands, (R)-3,3'-bis(tetramethylcyclopentadienyl)-2,2'-bismethoxy-1,1'-bisnaphthalene (1) and (R)-3-tetramethylcyclopentadienyl-2,2'-bismethoxy-1,1'-bisnaphthalene (2), were synthesized by reaction of (R)-3,3'-dilithium-2,2'-bismethoxy-1,1'-bisnaphthalene with 2,3,4,5-tetramethyl-2-cyclopentenone at room temperature. Treatment of the pre-ligands 1 and 2 with butyllithium and Me3SiCl first, and subsequently with TiCl4 (2 and 1 equiv for 1 and 2, respectively) afforded a binuclear complex (R)-3,3'-bis[(tetramethylcyclopentadienyl) trichlorotitanium]-2,2'-bismethoxy-1,1'-bisnaphthalene (3) and a mononuclear complex (R)-3-(tetramethylcyclopentadienyl)trichlorotitanium-2,2'-bismethoxy-1,1'-bisnaphthalene (4) in moderate yields. Complexes 3 and 4 were further converted into constrained geometry complexes (R)-1,1'-bis{2,2'-naphthoxy-3,3'-bis[(tetramethylcyclopentadienyl)dibromotitanium]} (5) and (R)-1-(2-naphthoxy)-1'-(2'-naphthol)-3-(tetramethylcyclopentadienyl)dibromotitanium (6) by treatment with BBr3. The pre-ligands 1 and 2 were characterized by H-1 and C-13 NMR and high resolution mass spectroscopy (HRMS), and the new titanium complexes 3-6 were characterized by H-1 and C-13 NMR and elemental analyses. Molecular structures of 4, 5, and 6 were determined by single-crystal X-ray diffraction analysis. Complexes 4, 5, and 6 all have a pseudo-octahedral coordination environment and adopt a three-legged piano stool geometry around the titanium atom in their solid state structures. When activated with (AlBu3)-Bu-i and Ph3CB(C6F5)(4), the chiral complexes 5 and 6 show moderate catalytic activities for propylene, 1-hexene, and 5-ethylidene-2-norbornene (ENB) polymerization and ethylene/1-hexene copolymerization. The polymers produced by the chiral 5/(Bu3Al)-Bu-i/Ph3CB(C6F5)(4) catalyst system from the 1-hexene, and ENB polymerization and ethylene/1-hexene copolymerization with high comonomer contents exhibit optical activity [4].
References
[1] Fendrick C M, Schertz L D, Day V W, et al. Manipulation of organoactinide coordinative unsaturation. Synthesis, structures, and reactivity of thorium hydrocarbyls and hydrides with chelating bis (tetramethylcyclopentadienyl) ancillary ligands[J]. Organometallics, 1988, 7(8): 1828-1838.
[2] Jacoby D, Keller F. One-step catalytic cyclocondensation process for the preparation of substituted 2-cyclopenten-1-ones from enones and aldehydes[P]. PCT Int. Appl., 2006051503, 2006.
[3] Chen);
You may like
See also
Lastest Price from 2,3,4,5-TETRAMETHYL-2-CYCLOPENTENONE manufacturers

US $36.48/KG2023-09-21
- CAS:
- 54458-61-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 15MT

US $36.47/KG2023-09-21
- CAS:
- 54458-61-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 15MT