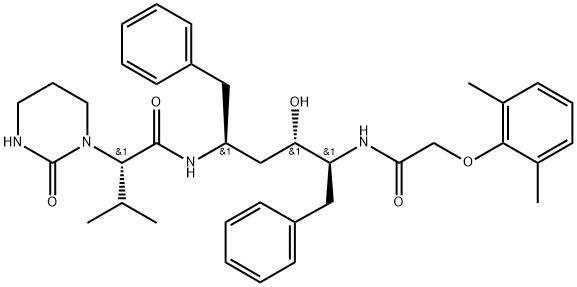
Side effects of Lopinavir
Lopinavir (formerly known as ABT-378) is an HIV-1-specific protease inhibitor engineered specifically to address the shortcomings of earlier agents in the protease inhibitor class. It is the only protease inhibitor to be coformulated with ritonavir, an oldergeneration protease inhibitor commonly exploited for its pharmacologic enhancing effect (Sham et al., 1998). Ritonavir, a potent inhibitor of hepatic cytochrome P450 (CYP) 3A isoenzyme, decreases metabolism and increases the plasma levels of lopinavir.

Uses
Lopinavir–ritonavir is a potent, well-tolerated agent which has demonstrated activity in antiretroviral-naive and -experienced HIV-1-infected patients; clinical trials have demonstrated durability in antiretroviral-naive patients for greater than five and seven years of follow-up. Until recent large studies demonstrated similar efficacy with other ritonavir-boosted protease inhibitors, lopinavir–ritonavir was considered the preferred protease inhibitor in US national guidelines.
Mechanism of action
Like all protease inhibitors, lopinavir–ritonavir exerts its antiretroviral effect by competitive inhibition of the HIV protease enzyme, whose normal function is cleavage of the Gag–Pol gene product. Inhibition of this late step in the viral life cycle results in the production of replication-incompetent virions.
Bioavailability
Lopinavir, when used alone, produces inadequate systemic concentrations owing to extensive metabolism by the cytochrome P450 (CYP) 3A isoenzymes. Since ritonavir potently inhibits CYP3A4/5, its coadministration is used to enhance the plasma levels of lopinavir. Studies in HIV-infected patients have shown that lopinavir–ritonavir administered in a dose of 400/100 mg twice daily yields mean steadystate lopinavir plasma concentrations 15- to 20-fold higher than those of ritonavir. Furthermore, the plasma levels of ritonavir are less than 7% of those obtained with the standard ritonavir dose of 600 mg twice daily. Combination drug activity studies with lopinavir and other protease inhibitors or reverse transcriptase inhibitors have not been completed.
The pharmacokinetics of once-daily lopinavir–ritonavir 800/200 mg (using the capsule formulation which is no longer available) has also been evaluated in antiretroviral-naive, HIV-infected subjects. Compared with the twice-daily dosing, the once-daily dosing produced more variable pharmacokinetics median steady state Cmax, minimal concentration (Cmin), and AUC were 12.8 (10.3–17.2) mg/ml, 1.34 (0.58–3.25) mg/ml h, and 143 (116–214) mg/ml h, respectively. Although once-daily dosing resulted in sufficient AUCs over the 24-hour interval, its variability is higher and Cmin values are significantly lower than the twice-daily dosage, and at times it can drop below the recommended plasma concentration of 1.0 mg/ml.
Side effects
Lopinavir–ritonavir is generally well tolerated. The most common adverse events in clinical trials are gastrointestinal, including nausea, vomiting, abdominal pain, dyspepsia, flatulence, anorexia, and especially diarrhea. A pooled analysis demonstrated that r6% of subjects receiving lopinavir–ritonavir in early clinical trials discontinued lopinavir–ritonavir because of drug-related toxicity. The incidence of diarrhea has been between 5% and 27% in early licencing studies of the lopinavir–ritonavir soft-gel capsules, and is significantly improved with the new tablet formulation of lopinavir–ritonavir.
Body habitus changes, including peripheral lipoatrophy and visceral adiposity, have been described in patients receiving lopinavir–ritonavir, but a direct correlation between lopinavir–ritonavir use and body habitus changes has not been demonstrated to date.
Severe adverse events
Severe (grade 3–4) adverse events related to the use of lopinavir– ritonavir are rare. Immediately prior to the licencing of lopinavir– ritonavir, it was used for approximately 6.5 months in over 4700 patients in four large international expanded access programs (EAP). In the Canadian, Italian, and Spanish arms of the EAP, toxicities included pancreatitis (o0.8%), hyperlipidemia (o0.4%), hepatitis (o0.4%), fever (o0.3%), myocardial infarction, diarrhea, dehydration, convulsion, allergic reaction, nausea, vomiting, lactic acidosis, abnormal liver function tests, hepatic failure, Cushing's syndrome, and rash.
Protease inhibitors have also been associated with a modest increase in risk of myocardial infarction related to duration of protease inhibitor exposure, although this risk is partially mitigated by the use of lipidlowering agents.
);You may like
Lastest Price from Lopinavir manufacturers

US $0.00-0.00/Kg/Bag2024-05-23
- CAS:
- 192725-17-0
- Min. Order:
- 0.1Kg/Bag
- Purity:
- USP
- Supply Ability:
- 20tons

US $26.00-20.00/kg2024-01-08
- CAS:
- 192725-17-0
- Min. Order:
- 1kg
- Purity:
- 99.50%
- Supply Ability:
- 30tons