Side effects of Fosamprenavir
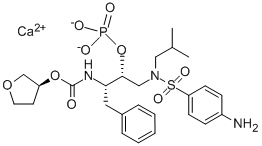
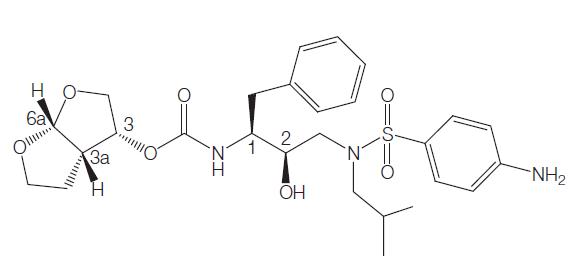
Fosamprenavir is a sulfa-containing calcium phosphate ester prodrug of amprenavir. Like other HIV-1 protease inhibitors, amprenavir binds to the active site of the HIV-1 protease. This prevents the processing of viral Gag and Gag–Pol polyprotein precursors, resulting in the formation of immature noninfectious viral particles.
Formerly called GW433908 or 908, fosamprenavir was developed to overcome the high pill burden and interpatient variable pharmacokinetics of the amprenavir soft-gelatin capsule formulation (Agenerases). The water solubility of fosamprenavir permitted the formulation of a tablet that does not contain the lipid excipients, which may have been responsible for some of the gastrointestinal adverse effects of Agenerase.
Mechanism of action
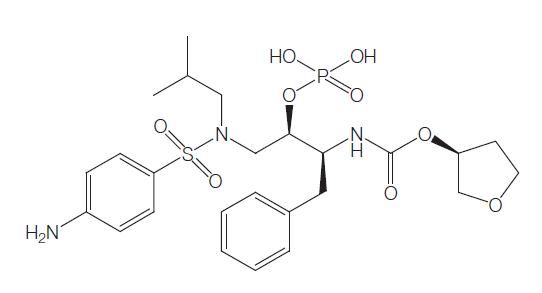
Fosamprenavir is the oral prodrug of amprenavir, an aspartic protease inhibitor that inhibits processing of viral Gag and Gag–Pol polyprotein precursors, resulting in the formation of immature noninfectious viral particles similar to other protease inhibitors.
Bioavailability
Fosamprenavir is 99% hydrolyzed to organic phosphate and amprenavir by cellular proteases in the gut epithelium. The main enzyme responsible for this conversion is alkaline phosphatase. The proportion of orally administered fosamprenavir that gets into the systemic circulation is negligible. In animal studies, less than 1% of an oral fosamprenavir dose was detected in the portal vein. In humans, plasma fosamprenavir AUC and maximum concentration (Cmax) were o0.6% and o1.6% of the corresponding plasma amprenavir values, respectively.
Amprenavir produced from the breakdown of fosamprenavir enters the systemic circulation mainly by passive diffusion. Amprenavir is also a substrate for P-glycoprotein, a drug transporter that may play a role in amprenavir absorption. Amprenavir is present in measurable quantities in the systemic circulation by 0.25 hours after an oral dose of fosamprenavir, reaching Cmax approximately 1.5–2.5 hours after single or repeated oral dosing.
Drug interactions
The drug interactions seen with fosamprenavir or fosamprenavirritonavir are due mainly to the effects of these agents on the CYP3A4 system. Amprenavir is essentially an inhibitor of CYP3A4, but it is also an inducer and a substrate for the same enzyme. The plasma concentrations of some drugs can be increased to potentially lifethreatening levels if co-administered with fosamprenavir. Such drugs (astemizole, cisapride, dihdroergotamine, midazolam, pimozide, bepridil, terfenadine, lovastatin, simvastatin, and triazolam) should not be administered concurrently with fosamprenavir.
For other drugs, such as calcium channel blockers, atorvastatin, and phosphodiaesterase inhibitors (sildenafil, tadalafil, and verdenafil), concurrent administration is permissible provided lower doses are used and there is close monitoring for toxicity.
Side effects
Coadministration of 200 mg of ritonavir with fosamprenavir is associated with higher rates of gastrointestinal intolerance and lipid elevation compared with fosamprenavir alone, making lower doses of ritonavir attractive.
Fosamprenavir has been generally well tolerated and safe. The reported side-effects are similar to others in the protease inhibitor drug class. Typically, the reported adverse effects are mild and self-limited. The most common moderate to severe adverse events are diarrhea, rash, nausea, vomiting, and headache.
In the NEAT trial, approximately one-third of patients treated with fosamprenavir 1400 mg twice daily developed at least one grade 2–4 adverse event by week 48. A slightly higher proportion of patients (41%) in the SOLO trial (fosamprenavir– ritonavir 1400/100 mg daily) had at least one grade 2–4 adverse event by week 48. Premature treatment discontinuation as a result of an adverse event occurred in 5% (9/ 166) of patients in the fosamprenavir arm of the NEAT trial and 6% (27/434) of patients in the fosamprenavir–ritonavir 700/100 mg arm of the KLEAN trial. Importantly, both studies included the nucleoside reverse transcriptase inhibitor backbone of abacavir–lamvudine, and many of the treatment discontinuations were due to abacavir hypersensitivity and not adverse effects related to fosamprenavir.
You may like
Lastest Price from Fosamprenavir calcium manufacturers

US $5.00-450.00/G2022-11-05
- CAS:
- 226700-81-8
- Min. Order:
- 1G
- Purity:
- 99.99%
- Supply Ability:
- 1000kg

US $15.00-10.00/KG2021-07-13
- CAS:
- 226700-81-8
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton