Olivetol: Reaction / Application on synthetic works
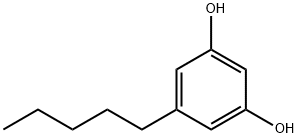
Olivetol, also known as 5-pentylresorcinol or 5-pentyl-1,3-benzenediol, is an organic compound found in certain species of lichen; it is also a precursor in various syntheses of tetrahydrocannabinol.
Example 1
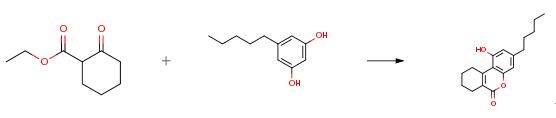
Olivetol (49.5 g, 261 mmol) and ethyl 2-oxocyclohexanecarboxylate (47.7 ml, 285 mmol) were initially introduced in toluene and, after addition of 28.8 ml (310 mmol) of phosphoryl chloride, were stirred at room temp. for 38 h. The reaction mixture was slowly poured into 400 ml of water and stirred vigorously for 30 min. The precipitated yellow solid was filtered off and washed with water and toluene. The pale-yellow solid was taken up in hot ethyl methyl ketone, washed with aqueous saturated sodium hydrogen carbonate soln. solution and sat. sodium chloride solution, dried over sodium sulfate, filtered and evaporated in vacuo. Crystallization of the crude product from acetonitrile/toluene gave 32.7 g (82 percent) of 1-hydroxy-3-pentyl-7,8,9,10-tetra-hydrobenzo[c]chromen-6-one as colourless crystals.
Example 2
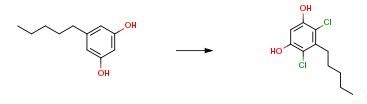
To a cold (0° C.) solution of 5-pentylbenzene-1,3-diol (25 g, 139 mmol) in dichloromethane (250 ml) was slowly added sulfuryl chloride (46.8 g, 347 mmol, 28.1 ml) over a 30 min period. The resulting mixture was stirred over night while slowly warming to room temperature. The reaction mixture was quenched with 1M aq. NaOH (150 mL) and subsequently stirred for 15 minutes. The formed slurry was diluted with extra DCM (100 mL) and then acidified to pH about 3 with 3 M aq. HCl. The layers were separated and the aqueous phase was extracted once with DCM (150 mL). The combined organic layers were dried on Na2SO4 and all volatiles evaporated in vacuo using a rotary evaporator. Un-purified 31.1 g brown/yellow oil. The oil was dissolved in heptane (100 mL) and stored the solution at 4° C. The formed crystals were filtered off and then dried on filter by air stream. Yield: 22.4 g slightly yellow solid (65percent yield).
Example 3

A solution of the appropriate resorcinol 1 in methane sulfonic acid and 3,3-dimethylacrylic acid were added to a suspension of P2O5 in methane sulfonic acid under N2 atmosphere. The reaction mixture was heated following three different procedures: a) irradiation by microwave at 70 °C sealed reactor for 10 minutes; b) irradiation by microwave at 70 °C in a sealed reactor for 20minutes; c) irradiation by microwave at 70° C in a sealed reactor for 1 hour; d) conventional heating at 70 °C for about 12 hours. The reaction mixture was poured onto water/ice and then extracted with DCM. The organic layer was dried over anhydrous MgSO4. Solvent was removed under reduced pressure. The crude compounds were purified and isolated by chromatography on silica gel (hexane/ EtOAc).
References
1. Taugerbeck A, Pauluth D, Kretschmer B, Wurziger H, Jansen A, Maillard D. Oxaphenanthrene Derivatives. US2008/312224[P], 2008, A1, Page column 9
2. Noramco I, Dialer L, Petrovic D, Weigl U. Process for the Production of Cannabidiol And Delta-9-Tetrahydrocannabinol. US2017/8868[P], 2017, A1, Paragraph 0268; 0269.
3. Morales P, Azofra LM, Cumella J, Hernandez-Folgado L, Roldan M, Alkorta I, Jagerovic N. Preparation of 2,2-dimethylchroman-4-ones from 5-alkyl-substituted resorcinols: Microwave-assisted synthesis and theoretical calculations[J]. Arkivoc, 2014, 2014(2):319 - 332.
You may like
Related articles And Qustion
See also
Lastest Price from Olivetol manufacturers

US $1.00/g2024-06-02
- CAS:
- 500-66-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $0.00-0.00/KG2024-05-31
- CAS:
- 500-66-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5000