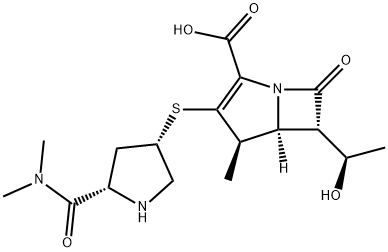
Meropenem: Antimicrobial Activity, Susceptibility, Administration and Dosage, Clinical Uses etc.
Meropenem is a broad-spectrum parenteral carbapenem with antimicrobial activity against a wide range of Gram-negative and Grampositive bacteria, including anaerobes. As a consequence of emerging multidrug resistance, especially in Gram-negative organisms, the clinical reliance on meropenem and other carbapenems is increasing, especially in the intensive care unit setting. Compared with imipenem, meropenem has slightly greater in vitro activity toward many Gramnegative organisms and, conversely, slightly less activity toward Grampositive organisms (Edwards et al., 2005; Turner, 2007). Similar to imipenem, meropenem is a thienamycin derivative that was licensed by the US Food and Drug Administration (FDA) in 1996 (Hellinger and Brewer, 1999).
Meropenem contains a four-member lactam ring fused to a fivemember thiazolidinic secondary ring through the nitrogen and adjacent tetrahedral carbon atom (see Figure 37.1) (Nicolau, 2008). The unique structural component that differentiates the carbapenems and influences antimicrobial activity and stability toward b-lactamases is the side chains attached to this two-ring structure. Meropenem has a pyrrolidinyl substituent at the 2 position in its side chain (Figure 37.1), which is thought to provide the improved Gram-negative activity (Zhanel et al., 2007). Unlike imipenem, meropenem is intrinsically stable to human renal dehydropeptidate-1 (DHP-1) and therefore does not require coadministration with an enzyme inhibitor, such as cilastatin (Fukasawa et al., 1992). The most important phamacodynamic parameter predicting in vivo efficacy of meropenem, as with other b-lactams, is the time that the plasma drug concentration is maintained above the minimim inhibitory concentration (TWMIC) (Nicolau, 2008). It is rapidly bactericidal. Meropenem is formulated as a mixed powder of meropenem and dried sodium carbonate available in a vial for reconstitution and injection.
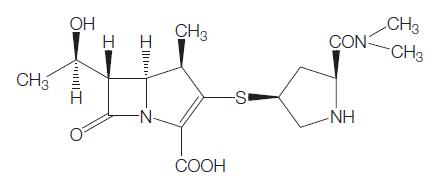
Chemical structure of meropenem.
ANTIMICROBIAL ACTIVITY
a. Routine susceptibility
Meropenem has very broad spectrum activity against a variety of Gram-positive and Gram-negative bacteria (see Tables 37.1, 37.2, and 37.3).
Gram-positive aerobic bacteria
Meropenem has activity against aerobic Gram-positive organisms (Table 37.1); however, there are exceptions, including methicillinresistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Enterococcus faecium. As already mentioned, imipenem has greater in vitro activity toward many Gram-positive organisms; however, the clinical effect of this difference remains to be determined. Meropenem also has activity against Bacillus spp., Listeria monocytogenes (Marco et al., 2000), Erysipelothrix rhusiopathiae (Kayser et al., 1989), and many Corynebacterium spp., with the exception of C. jeikeium, an organism that is often resistant to multiple antibiotics and can cause serious infection, such as bacteremia, endocarditis, foreign body infections, and wound infections.
Gram-negative aerobic bacteria
This group of organisms has become one of the most formidable challenges to antibiotic activity over the last decade. Meropenem remains highly active toward the majority of aerobic Gram-negative bacteria (Table 37.2), with stability against many problematic b-lactamases, including extended-spectrum b-lactamases (ESBLs) and Amp-C type b-lactamases. Meropenem is considered first-line therapy for serious infections caused by such organisms (Paterson et al., 2004) as its in vitro potency is 4- to 16-fold greater than that of imipenem (Turner, 2007). Also, meropenem was reported to have a 2- to 8-fold greater in vitro potency toward ESBLs than ertapenem when ertrapenem MICs were Z2mg/l (Rhomberg et al., 2007).
Despite meropenem not being a first-line agent for many Gramnegative organisms, such as Haemophilus influenzae, Moraxella catarrhalis, and the Neisseria spp., knowledge of its activity is relevant in certain clinical situations (see below under 7f. Bacterial Meningitis). Other Gram-negative organisms that are often susceptible to meropenem include Campylobacter jejuni, Hafnia alvei, Pasteurella multocida, and Yersinia enterocolitica.
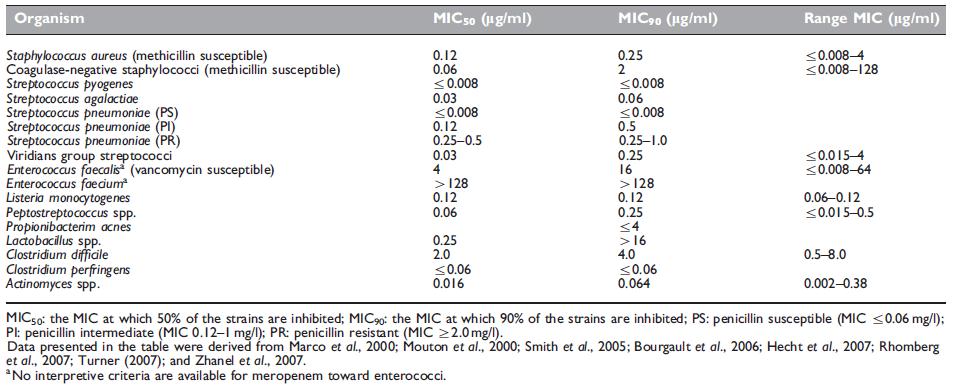
Table 37.1 Antimicrobial activity of meropenem against Gram-positive organisms.
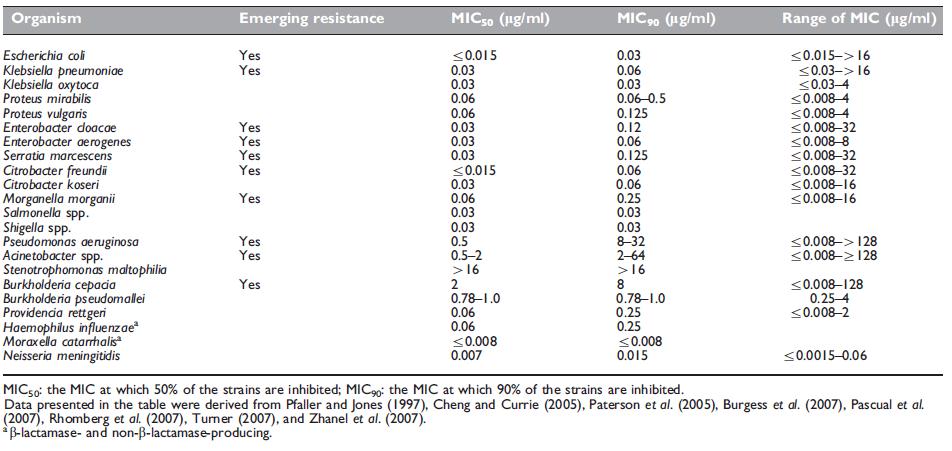
Table 37.2 Antimicrobial activity of meropenem against aerobic Gram-negative organisms.
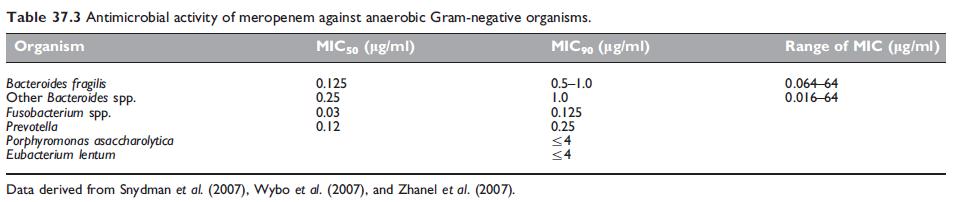
Antimicrobial activity of meropenem against anaerobic Gram-negative organisms.
Anaerobic bacteria
The carbapenems as a class have excellent in vitro activity against anaerobic organisms (Table 37.1); however, the exceptions for Gram-positive anaerobes include Clostridium difficile and Lactobacillus.
Meropenem remains highly potent against the vast majority of anaeorobic Gram-negative organisms (Table 37.3), with low rates of resistance being reported (Rhomberg et al., 2007; Snydman et al., 2007). Approximately 2–4% of Bacteroides fragilis strains carry a metallo-b-lactamase, designated as CcrA or CfiA, which may confer resistance (Thompson and Malamy, 1990; Yamazoe et al., 1999).
b. Emerging resistance and crossresistance
Given the potency and breadth of activity of the carbapenems and the paucity of new antimicrobials in the pipeline, cautious and prudent use of this class of antimicrobial is imperative. Despite meropenem retaining excellent activity against the majority of Gram-negative organisms, carbapenem resistance is emerging worldwide, particularly in nonfermenting Gram-negative organisms such as P. aeruginosa and A. baumannii, and to a lesser degree Enterobacteriaceae (Van Looveren et al., 2004; Gaynes et al., 2005; Gales et al., 2006; Peleg et al., 2006; Nicasio et al., 2008). Broadly speaking, resistance to the carbapenems is mediated by either enzymatic degradation from b-lactamases, membrane-based changes, which include reduced drug permeability, increased drug efflux, or a combination of the two, or alterations in target penicillin-binding proteins (PBPs). Most often, multiple mechanisms are responsible for the final resistance phenotype (Bou et al., 2000; Fernandez-Cuenca et al., 2003; Quale et al., 2006). b-Lactamases that are able to hydrolyze carbapenems are known as carbapenemases and include both serine b-lactamases (molecular class A and D) and metallo-b-lactamases (molecular class B) (Queenan and Bush, 2007).
MECHANISM OF DRUG ACTION
Similar to other b-lactams, meropenem exhibits bactericidal activity by binding to and inactivating PBPs, which are responsible for the elongation and cross-linking (transpeptidation) of peptidoglycan strands making up the bacterial cell wall (Nicolau, 2008). Thus, impaired cell wall synthesis ensues, leading to inhibited growth and cell lysis. The affinity of meropenem for the various PBPs differs between Gram-positive and Gram-negative organisms preferentially it binds to PBPs 1, 2, and 4 in Gram-positive organisms and to PBPs 2, 3, and 4, as well as showing strong affinity for PBPs 1a and 1b, in Gramnegative organisms (Sumita and Fukasawa, 1995; AstraZeneca, 2005; Zhanel et al., 2007). The ability of meropenem to bind to all three essential PBPs may explain its rapid bactericidal effect (Satta et al., 1995).
MODE OF DRUG ADMINISTRATION AND DOSAGE
a. Adults
Meropenem is available only in parenteral form. In adults with normal renal function, meropenem is typically dosed at 500–2000 mg, depending upon the infection type (see below), every 8 hours as an intravenous infusion over 15–30 minutes. Doses of 1000 mg or less may also be administered as an intravenous push over 3–5 minutes when reconstituted with sterile water at a concentration of up to 50 mg/ml (AstraZeneca, 2005). Infusion vials are reconstituted with 0.9% normal saline or 5% dextrose solution and should be used fresh whenever possible. Overall, meropenem stability is improved if reconstituted in 0.9% normal saline compared with 5% dextrose, being stable for 2–4 hours (4 hours if stored in a plastic i.v. bag at concentration o20 mg/ml) compared with 1 hour at room temperature, respectively (AstraZeneca, 2005). This limits the ability to use meropenem as a continuous infusion. However, meropenem stability is prolonged if kept at 4 1C, and investigators have stored delivery pumps in a cold pouch with success (Kuti et al., 2004). Each 500-mg dose contains 45.1 mg sodium as sodium carbonate (1.96 mEq).
b. Newborn infants and children
Dosing recommendations for children Z3 months are 10, 20, or 40 mg/kg every 8 hours (maximum dose is 2000 mg every 8 hours) depending on the infection type. Complicated skin and skin structure infections require 10 mg/kg per dose (maximum of 500 mg) every 8 hours and intra-abdominal infections require 20 mg/kg per dose (maximum of 1000 mg) every 8 hours (AstraZeneca, 2005). Recommendations for febrile neutropenia vary according to weight: o50 kg, dose at 20 mg/kg per dose (maximum of 1000 mg) every 8 hours; W50 kg, use adult dosing (Cometta et al., 1996). In the case of bacterial meningitis, the recommended dose increases to 40 mg/kg per dose (maximum of 2000 mg) every 8 hours (Odio et al., 1999).
TOXICITY
Overall, meropenem exhibits a favorable safety profile (Table 37.7). No differences have been observed between meropenem administered as a 5-minute bolus injection or as a 30-minute infusion (Hellinger and Brewer, 1999; Norrby and Gildon, 1999). An updated safety review of over 6000 patient exposures reported drug-related clinical adverse events to be low (no adverse event occurred in W3% of patient exposures) (Linden, 2007). The most common adverse events included diarrhea (2.5%), rash (1.4%), and nausea/vomiting (1.2%). These adverse events were considered mild to moderate and led to discontinuation of therapy in 1.4–2.5% of patients (Linden, 2007; Zhanel et al., 2007). The most common laboratory adverse events were increases in liver enzymes, including alanine aminotransferase (3.7%), aspartate aminotransferase (2.9%), and alkaline phosphatase (1.2%), and eosinophilia (o2% of patients) (Linden, 2007). Linden (2007) also reported increases in serum creatinine and urea in o1% of patients.
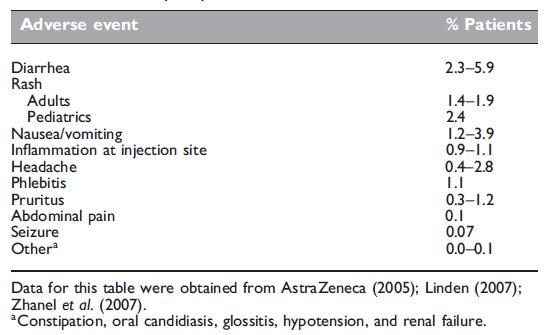
Overall incidence of meropenem-related adverse events
listed in order of frequency.
CLINICAL USES OF THE DRUG
Given meropenem’s broad antimicrobial spectrum, it has a wide range of clinical uses, most often in serious hospital-acquired infections. As mentioned, judicious and careful use of this class of antimicrobial is critical to prevent the widespread emergence of carbapenem resistance. This section will concentrate on randomized, clinical trials assessing meropenem efficacy – these are summarized in Table 37.8.
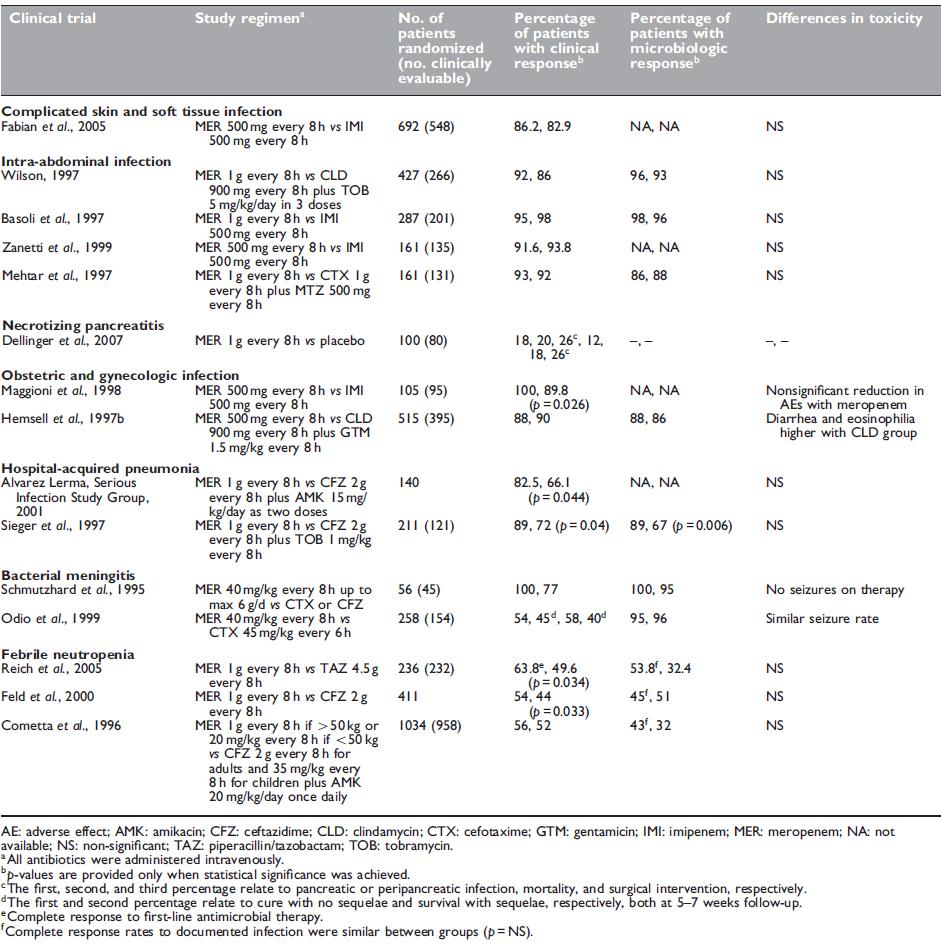
Table 37.8 Outcome data from clinical trials with meropenem.
a. Skin and soft tissue infection
A prospective, randomized, double-blind, multicenter study involving hospitalized patients with complicated skin and skin structure infections was performed to compare the efficacy of meropenem with imipenem–cilastatin, both given at 500 mg every 8 hours (Fabian et al., 2005). Patients included those with complicated cellulitis, complex abscesses, perirectal abscesses, and skin infections requiring intravenous antimicrobials, hospitalization, and surgical intervention. The primary efficacy end point was clinical outcome at a follow-up visit 7–14 days after the cessation of therapy. A total of 1076 patients were enrolled, with 548 being clinically evaluable (261 for meropenem and 287 for imipenem–cilastatin).
b. Intra-abdominal infection
Several randomized, controlled trials show that meropenem has excellent efficacy for intra-abdominal infections (see Table 37.8). Meropenem has been compared with imipenem (Brismar et al., 1995; Colardyn and Faulkner, 1996; Basoli et al., 1997; Zanetti et al., 1999), clindamycin and tobramycin (Wilson, 1997), and cefotaxime and metronidazole (Mehtar et al., 1997), with similar efficacy reported. In patients with advanced appendicitis (gangrenous or perforated), meropenem showed small yet significant benefits compared with tobramycin and clindamycin, including reduced postoperative fever, duration of antibiotics, and hospital stay (Berne et al., 1996). Given the higher likelihood of drug-resistant pathogens in healthcareassociated intra-abdominal infections (most often postoperative), meropenem constitutes an excellent single agent to cover against Gram-negative aerobic and anaerobic organisms.
c. Acute pancreatitis
The use of carbapenems for the prophylaxis of infection in patients with acute necrotizing pancreatitis has been controversial. A Cochrane review performed in 2006 concluded that antibiotic prophylaxis appeared to be associated with significantly less mortality with no reduction in pancreatic infection, and that further doubleblind randomized controlled trials are required (Villatoro et al., 2006).
d. Obstetric and gynecologic infections
Several comparative studies have been performed in patients with gynecologic or obstetric infections to compare meropenem with imipenem–cilastatin (Maggioni et al., 1998) or clindamycin plus gentamicin (Hemsell et al., 1997a). As expected, the clinical and bacteriologic response rates were high (Table 37.8). As mentioned, meropenem is a pregnancy category B drug.
e. Respiratory infection
Despite several studies showing the efficacy of meropenem for the treatment of community-acquired pneumonia (Bartoloni et al., 1999; Romanelli et al., 2002), it is not recommended as a first-line agent. The exception to this is in tropical regions such as Southeast Asia and northern Australia, where B. pseudomallei is an important cause of community-acquired pneumonia. Meropenem is a therapeutic option (1 g or 25 mg/kg every 8 hours) (Cheng et al., 2004) and is often used for severe cases.
f. Bacterial meningitis
Meropenem has excellent activity against most organisms causing community-acquired bacterial meningitis and has good CSF penetration.
Rather than being a first-line agent, it is used as an alternative therapy (Tunkel et al., 2004) at a dose of 40 mg/kg up to 2 g every 8 hours. The efficacy of meropenem compared with cephalosporins in bacterial meningitis has been demonstrated in adults and children in several prospective, randomized trials (Schmutzhard et al., 1995; Odio et al., 1999). No differences were observed in clinical cure or neurologic sequelae. No excess seizure activity was attributed to meropenem.
Clinical situations in which meropenem may be used in communityacquired bacterial meningitis include patients who are intolerant of penicillins and/or cephalosporins, treatment of penicillin-intermediate S. pneumoniae (MIC, 0.1–1.0mg/l), and as an alternative treatment for L. monocytogenes meningitis (Tunkel et al., 2004).
g. Febrile neutropenia
Several prospective, randomized, clinical trials have been performed in both adults and pediatric populations that confirm the efficacy of meropenem in patients with febrile neutropenia (Table 37.8). Meropenem monotherapy (1 g every 8 hours) has been compared with piperacillin–tazobactam (Reich et al., 2005), ceftazidime (Lindblad et al., 1998; Feld et al., 2000; Vandercam et al., 2000; Fleischhack et al., 2001), ceftazidime plus amikacin (Cometta et al., 1996; Behre et al., 1998), cefepime (Oguz et al., 2006), and imipenem– cilastatin (Shah et al., 1996).
References
Aaron SD, Ferris W, Henry DA et al. (2000). Multiple combination bactericidal
antibiotic testing for patients with cystic fibrosis infected with Burkholderia
cepacia. Am J Resp Crit Care Med 161: 1206.
Alvarez Lerma F, the Serious Infection Study Group (2001). Efficacy of
meropenem as monotherapy in the treatment of ventilator-associated
pneumonia. J Chemother 13: 70.
Alvarez C, Ramos JM, San Juan R et al. (2005). Risk of superinfection related to
antibiotic use. Are all antibiotics the same? Rev Esp Quimioter 18: 39.
Anaya DA, Dellinger EP (2007). Necrotizing soft-tissue infection: diagnosis and
management. Clin Infect Dis 44: 705.
Burgess DS, Frei CR, Lewis JS et al. (2007). The contribution of
pharmacokinetic-pharmacodynamic modelling with Monte Carlo simulation
to the development of susceptibility breakpoints for Neisseria meningitidis.
Clin Microbiol Infect 13: 33.
Capitano B, Nicolau DP, Potoski BA et abl. (2004). Meropenem administered as a
prolonged infusion to treat serious gram-negative central nervous system
infections. Pharmacotherapy 24: 803.
Cercenado E, Marin M, Sanchez-Martinez M et al. (2007). In vitro activities
of tigecycline and eight other antimicrobials against different Nocardia
species identified by molecular methods. Antimicrob Agents Chemother 51:
1102.
Drusano GL, Hutchison M (1995). The pharmacokinetics of meropenem. Scand
J Infect Dis 96: 11.
Edwards SJ, Emmas CE, Campbell HE (2005). Systematic review comparing
meropenem with imipenem plus cilastatin in the treatment of severe
infections. Curr Med Res Opin 21: 785.
Fabian TC, File TM, Embil JM et al. (2005). Meropenem versus imipenemcilastatin
for the treatment of hospitalized patients with complicated skin
and skin structure infections: results of a multicenter, randomized, doubleblind
comparative study. Surg Infect 6: 269.
Granai F, Smart HL, Triger DR (1992). A study of the penetration of
meropenem into bile using endoscopic retrograde cholangiography.
J Antimicrob Chemother 29: 711.
Hanberger H, Nilsson LE (1994). Retain intermittent dosing of carbapenems.
Antimicrob Agents Chemother 38: 159.
Harrison MP, Haworth SJ, Moss SR et al. (1993). The disposition and metabolic
fate of 14C-meropenem in man. Xenobiotica 23: 1311.
You may like
Lastest Price from Meropenem manufacturers

US $0.00/kg2024-04-29
- CAS:
- 96036-03-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $15.00/kg2024-04-27
- CAS:
- 96036-03-2
- Min. Order:
- 1kg
- Purity:
- 99.912%
- Supply Ability:
- 10ton