-
外観
無色澄明の液体
-
溶解性
水に微溶。エタノール、エーテルと混和。
-
解説
ベンゼン,benzole.C6H6(78.11).石油改質中に,また石炭を乾留したときのガスおよびタール中に多量に含まれ,工業的にこれらから分離,精製する.すなわち,石油精製工業においては,ナフサを接触改質してベンゼン,トルエン,キシレンなどの芳香族炭化水素に富む改質油にかえ,スルホラン法,その他によって芳香族のみを抽出し,さらに分留してトルエン,キシレン,そのほかの留分と分ける.また,トルエンやキシレンの脱メチル水素化法で製造するときには,Cr2O3,Mn2O3,CoOなどの担持触媒による接触反応で処理し,メチル基をメタンとして除去して製造される.ベンゼンは,芳香族炭化水素の代表的な化合物であり,芳香族特有の香りをもつ,無色,揮発性の液体で,人体には有毒である.融点5.5 ℃,沸点80.1 ℃.d2540.8737.n25D1.4979.比熱容量1.734 J K-1 mol-1(21.8 ℃).蒸発熱392.6 J g-1(沸点).引火性が強く引火点-11.10 ℃.λmax 198,256 nm(ε 8000,230).エタノール,エーテルなど多くの有機溶媒に可溶,水に難溶.水29.6物質量% で沸点69.25 ℃(101 kPa)の最低共沸混合物をつくる.ベンゼンの構造は正六角形の平面であり,C-C0.1397 nm,C-H0.1084 nm,∠C-C-Cおよび∠H-C-Cはすべて120°である.ベンゼンは化学的に安定な化合物であるが,強力な試薬によって置換反応および付加反応を受ける.とくにニトロ化,ハロゲン化,スルホン化,あるいはフリーデル-クラフツ反応のような親電子置換反応は,ベンゼンの代表的な反応であり,これによってきわめて多種類の置換ベンゼン誘導体が合成され,これらは芳香族系合成化学,ならびにその工業の中間物として重要なものが多い.付加反応においては,塩素をラジカル的に付加させるとヘキサクロロシクロヘキサン(BHC)となり,水素を付加させるとシクロヘキサンが得られる.また,ニトロベンゼン,クロロベンゼンを経て多数のベンゼン誘導体に導き,染料,医薬,農薬などの中間物として用いられる.LD50 3800 mg/kg(ラット,経口).
-
用途
汎用試薬、溶剤、有機合成原料。
-
用途
高速液体クロマトグラフィーの溶離液及び溶離液の調製。
-
用途
本物質の主な用途は、純ベンゼンでは合成原料として染料、合成ゴム、合成洗剤、有機顔料、有機ゴム薬品、医薬品、香料、合成繊維(ナイロン)、合成樹脂(ポリスチレン、フェノール、ポリエステル)、食品(コハク酸、ズルチン)、農薬(2,4-D、クロルピクリンなど)、可塑剤、写真薬品、爆薬(ピクリン酸)、防虫剤(パラジクロロベンゼン)、防腐剤(PCP)、絶縁油(PCD)、熱媒、溶剤級ベンゼンでは塗料、農薬、医薬品など一般溶剤、油脂、抽出剤、石油精製など、その他アルコール変性用である
-
用途
機器及び比色分析における低バックグラウンド抽出溶媒、高純度溶剤。
-
用途
汎用試薬、高純度を要する溶剤等。
-
用途
アミノ酸配列分析における溶剤。
-
用途
薄層及びペーパークロマトグラフィーの展開溶媒。
-
用途
分解ガソリン等から抽出される芳香族炭化水素で、スチレン、フェノール、シクロヘキサン等の原料として使用される他、各種化学品の原料、溶剤等に使用されます。
-
用途
精密分析、超高純度溶剤としての個人専用試薬。
-
用途
食品及び水中等の農薬及びPCB定量における溶媒。
-
用途
各種化学製品の中間体製造のための出発原料として重要である。ベンゼンはアルキルベンゼン、フェノール、アニリン、スチレン、クロロベンゼン、ニトロベンゼン、無水マレイン酸などの合成原料であり、これらからさらに各種の樹脂、繊維、洗剤、染料、殺虫剤、爆薬、医薬品などが誘導される(図)。
-
使用上の注意
不活性ガス封入
-
性質と構造
特有のにおいをもつ無色透明で可燃性の液体で、煤(すす)の多い黒い煙をあげて燃える。その蒸気は有毒である。水には難溶だが、エタノール(エチルアルコール)やエーテルとは任意の割合で混じり合う。大気中のベンゼンは有害大気汚染物質と定められていて、長期間吸収すると造血器の障害をおこし、貧血などの原因になる。白血病などの癌(がん)性疾患を引き起こすともいわれている。
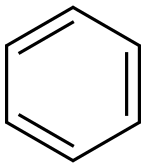
1825年にイギリスのM・ファラデーにより、鯨油の赤熱分解で得られたガスを凝縮させた液体中から最初に発見され、発見当時からその構造と化学的特性に関心が集まった。1865年、ドイツのF・A・ケクレが有名な亀甲(きっこう)形の六角環状説(ベンゼン環)を提案したが、最終的には1930年代になって、ようやくX線および電子線回折測定により正確な構造が決められた。それによると、ベンゼン環は1辺が0.1399ナノメートルの正六角形で、6本の炭素‐炭素(C-C)結合はまったく同等であり、6個のπ(パイ)電子が3本の二重結合に2個ずつ局在化しているのではなく、6個の炭素に平等に共有され、非局在化していることが証明された。ベンゼンの構造、性質が解明される過程において芳香族化合物の化学は発展し、この意味でも化学に果たした役割は非常に大きい。
-
説明
Benzene is a colorless, volatile, highly flammable liquid that is used extensively in the chemical industry and received wide interest in the early days of organic chemistry.

Because of its structure, benzene is a very stable organic compound. It does not readily undergo addition reactions. Addition reactions involving benzene require high temperature, pressure, and special catalysts. The most common reactions involving benzene involve substitution reactions. Numerous atoms and groups of atoms may replace a hydrogen atom or several hydrogen atoms in benzene. Th ree important types of substitution reactions involving benzene are alkylation, halogenation, and nitration. In alkylation, an alkyl group or groups substitute for hydrogen(s).
-
化学的特性
Benzene is a clear, volatile, colorless, highly flammable liquid with a pleasant, characteristic odor. It is an aromatic hydrocarbon that boils at 80.1 DC. Benzene is used as a solvent in many areas of industries, such as rubber and shoe manufacturing, and in the production of other important substances, such as styrene, phenol, and cyclohexane. It is essential in the manufacture of detergents, pesticides, solvents, and paint removers. It is present in fuels such as gasoline up to the level of 5%.
-
物理的性質
Clear, colorless to light yellow watery liquid with an aromatic, musty, phenolics or gasoline-like
odor. At 40 °C, an odor threshold concentration of 190 μg/L in air was determined by Young et al.
(1996). An odor threshold of 4.68 ppmv was determined by Leonardos et al. (1969). A detection
odor threshold concentration of 108 mg/m3 (34 ppmv) was reported by Punter (1983). The average
least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 0.072 and
0.5 mg/L, respectively (Alexander et al., 1982).
-
天然物の起源
Detectable levels of benzene have been found in a number of soft drinks that contain either a sodium or potassium benzoate preservative and ascorbic acid, and 'diet' type products containing no added sugar are reported to be particularly likely to contain benzene at detectable levels. Surveys carried out in the USA, the UK and Canada have all confirmed that a small proportion of these products may contain low levels of benzene. For example, in a survey of 86 samples analysed by the FDA between April 2006 and March 2007, only five products were found to contain benzene at concentrations above 5 ug kg-1. The levels found were in a range from approximately 10–90 ug kg-1. A survey of 150 UK-produced soft drinks by the Food Standards Agency (FSA) published in 2006 showed that four products contained benzene at levels above 10 ug kg-1, and the highest level recorded was 28 ug kg-1. However, it has been reported that higher levels may develop in these products during prolonged storage, especially if they are exposed to daylight.
Benzene may also be formed in some mango and cranberry drinks in the absence of added preservatives, because these fruits contain natural benzoates.
-
来歴
Benzene was discovered in 1825 by Michael Faraday (1791–1867), who identified it in a liquid residue from heated whale oil. Faraday called the compound bicarburet of hydrogen, and its name was later changed to benzin by Eilhardt Mitscherlich (1794–1863), who isolated the compound from benzoin (C14H12O2).
-
使用
Manufacturing of ethylbenzene (for styrene
monomer), dodecylbenzene (for detergents), cyclo-
hexane (for nylon), phenol, nitrobenzene (for ani-
line), maleic anhydride, chlorobenzene, diphenyl,
benzene hexachloride, benzene-sulfonic acid, and
as a solvent.
-
定義
ChEBI: Benzene is a six-carbon aromatic annulene in which each carbon atom donates one of its two 2p electrons into a delocalised pi system. A toxic, flammable liquid byproduct of coal distillation, it is used as an industrial solvent. Benzene is a carcinogen that also damages bone marrow and the central nervous system.
-
調製方法
Today benzene, which is a natural component of petroleum, is obtained from petroleum by several processes. Toluene hydrodealkylation involves mixing toluene (C6H5CH3) and hydrogen in the presence of catalysts and temperatures of approximately 500°C and pressures of about 50 atmospheres to produce benzene and methane: C6H5CH3 + H2 → C6H6 + CH4. Hydrodealkylation strips the methyl group from toluene to produce benzene. Toluene disproportionation involves combining toluene so that the methyl groups bond to one aromatic ring, producing benzene and xylene. Benzene can also be obtained from petroleum reforming in which temperature, pressure, and catalysts are used to convert petroleum components to benzene, which can then be extracted using solvents and distillation processes. Another source of benzene is pyrolysis gasoline or pygas.
-
反応性
Benzene reacts (1) with chlorine, to form (a) substitution products (one-half of the chlorine forms hydrogen chloride) such as chlorobenzene, C6H5Cl; dichlorobenzene, C6H4Cl2(1,4) and (1,2); trichlorobenzene, C6H3Cl3(1,2,4); tetrachlorobenzene (1,2,3,5); and (b) addition products, such as benzene dichloride C6H6Cl2; benzene tetrachloride, C6H6Cl4; and benzene hexachloride, C6H6Cl6. The formation of substitution products of the benzene nucleus, whether in benzene or its homologues, is favored by the presence of a catalyzer, e.g., iodine, phosphorus, iron; (2) with concentrated HNO3, to form nitrobenzene, C6H5NO2; 1,3- dinitrobenzene, C6H4(NO2)2 (1,3), 1,3,5-trinitrobenzene, C6H3(NO2)3 (1,3,5); (3) with concentrated H2SO4, to form benzene sulfonic acid, C6H5SO3H, benzene disulfonic acid, C6H4(SO3H)2(1,3), benzene trisulfonic acid, C6H3(SO3H)3 (1,3–5); (4) with methyl chloride plus anhydrous aluminum chloride (Friedel-Crafts reaction) to form toluene, monomethyl benzene, C6H5CH3; dimethyl benzene C6H4(CH3)2; trimethyl benzene, C6H3(CH3)3; (5) with acetyl chloride plus anhydrous aluminum chloride (Friedel-Crafts reaction) to form acetophenone (methylphenyl ketone), C6H5COCH3.
-
反応
芳香族性として知られているπ電子の非局在化によりベンゼン環が安定化していて壊れにくいので、ベンゼン誘導体はベンゼン環が失われる付加反応ではなく、反応の後にもベンゼン環が残る置換反応をおこしやすいという特徴がある。ベンゼンの置換反応としては、次の(1)~(4)などが代表的であり、これらの反応はいずれもベンゼン置換体の重要な合成法である。
(1)硝酸と硫酸混合物によるニトロベンゼンの生成(ニトロ化)
(2)発煙硫酸によるベンゼンスルホン酸の生成(スルホン化)(3)鉄粉を触媒とする塩素、臭素などのハロゲンとの反応によるクロロベンゼンやブロモベンゼンの生成(ハロゲン化)(4)塩化アルミニウムを触媒としたアルキル化によるアルキルベンゼンの生成、ならびにアシル化によるアセトフェノンなどの芳香族ケトンの生成(フリーデル‐クラフツ反応)。 これら(1)~(4)の反応では陽イオン試薬がπ電子(負電荷)をもつベンゼン環に反応しているので、芳香族求電子置換反応と総称されている。
しかし、次の(5)~(7)の例のように、高温、高圧といった強い反応条件下では、ベンゼン環への付加やベンゼン環が開環する反応もみられる。
(5)白金触媒やニッケル触媒を用いる水素化によるシクロヘキサンの生成
(6)接触気相酸化による無水マレイン酸などの合成(7)光を照射しながら塩素を反応させてBHC(ベンゼンヘキサクロリドの略、別名1,2,3,4,5,6-ヘキサクロロシクロヘキサン)を得る反応。
-
製法
石炭タールまたは石油から製造されている。しかし、芳香族炭化水素の石油中に含まれる量は少ないので、石油化学工業ではナフサの接触分解、リホーミングによりベンゼンのみならずトルエン、キシレンを含む炭化水素油をつくり、これから分留してベンゼンを製造する。またアルキルベンゼンのような高級同族体からは、脱アルキル化、水素化分解法によりベンゼンを得ている。
-
一般的な説明
Benzene appears as a clear colorless liquid with a petroleum-like odor. Flash point less than 0 °F. Less dense than water and slightly soluble in water. Hence floats on water. Vapors are heavier than air.
-
空気と水の反応
Highly flammable. Slightly soluble in water.
-
反応プロフィール
Benzene reacts vigorously with allyl chloride or other alkyl halides even at minus 70°C in the presence of ethyl aluminum dichloride or ethyl aluminum sesquichloride. Explosions have been reported [NFPA 491M 1991]. Ignites in contact with powdered chromic anhydride [Mellor 11:235 1946-47]. Incompatible with oxidizing agents such as nitric acid. Mixtures with bromine trifluoride, bromine pentafluoride, iodine pentafluoride, iodine heptafluoride and other interhalogens can ignite upon heating [Bretherick 5th ed. 1995]. Benzene and cyanogen halides yield HCl as a byproduct (Hagedorn, F. H. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.). The reaction of Benzene and trichloroacetonitrile evolves toxic chloroform and HCl gasses. (Hagedorn, F., H.-P. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.).
-
危険性
The acute toxicity of benzene is low. Inhalation of benzene can cause dizziness, euphoria, giddiness, headache, nausea, drowsiness, and weakness. Benzene can cause moderate irritation to skin and severe irritation to eyes and mucous membranes. Benzene readily penetrates the skin to cause the same toxic effects as inhalation or ingestion. The chronic toxicity of benzene is significant. Exposure to benzene affects the blood and blood-forming organs such as the bone marrow, causing irreversible injury; blood disorders including anemia and leukemia may result. The symptoms of chronic benzene exposure may include fatigue, nervousness, irritability, blurred vision, and labored breathing. Benzene is regulated by OSHA as a carcinogen (Standard 1910.1028) and is listed in IARC Group 1 ("carcinogenic to humans"). This substance is classified as a "select carcinogen" under the criteria of the OSHA Laboratory Standard.
-
燃焼性と爆発性
Benzene is a highly flammable liquid (NFPA rating = 3), and its vapors may travel a
considerable distance to a source of ignition and "flash back." Vapor-air mixtures are
explosive above the flash point. Carbon dioxide and dry chemical extinguishers
should be used to fight benzene fires.
-
化学反応性
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
-
工業用途
Benzene (C6H6, CAS No. 71-43-2) is an aromatic hydrocarbon compound used extensively in the chemical industry as an intermediate in the manufacture of polymers and other products. It is also a common atmospheric contaminant and is present in motor vehicle exhaust emissions and cigarette smoke.
In 1990, it was discovered by the USA soft drinks industry that benzene could be produced at low levels in certain soft drinks containing a benzoate preservative and ascorbic acid. Since benzene is a known human carcinogen, its presence in food and beverages is clearly undesirable.
-
安全性プロファイル
Confirmed human carcinogen producing myeloid leukemia, Hodgkin's dsease, and lymphomas by inhalation. Experimental carcinogenic, neoplastigenic, and tumorigenic data. A human poison by inhalation. An experimental poison by skin contact, intraperitoneal, intravenous, and possibly other routes. Moderately toxic by ingestion and subcutaneous routes. A severe eye and moderate sktn irritant. Human systemic effects by inhalation and ingestion: blood changes, increased body temperature. Experimental teratogenic and reproductive effects. Human mutation data reported. A narcotic. In industry, inhalation is the primary route of chronic benzene poisoning. Poisoning by skin contact has been reported. Recent (1 987) research indicates that effects are seen at less than 1 ppm. Exposures needed to be reduced to 0.1 ppm before no toxic effects were observed. Elimination is chiefly through the lungs.
-
職業ばく露
Benzene is used as a constituent in
motor fuels; as a solvent for fats; inks, oils, paints, plastics,
and rubber, in the extraction of oils from seeds and
nuts; in photogravure printing. It is also used as a chemical
intermediate. By alkylation, chlorination, nitration, and
sulfonation, chemicals, such as styrene, phenols, and
malefic anhydride are produced. Benzene is also used in
the manufacture of detergents, explosives, pharmaceuticals;
in the manufacture of cyclohexane and ethylbenzene;
and dye-stuffs. Increased concern for benzene as a significant
environmental pollutant arises from public exposure
to the presence of benzene in gasoline and the increased
content in gasoline due to requirements for unleaded fuels
for automobiles equipped with catalytic exhaust
converters.
-
発がん性
Benzene is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.
-
環境運命予測
Benzene is released to air primarily by vaporization and combustion emissions associated with its use in gasoline. Other sources are vapors from its production and use in manufacturing other chemicals. In addition, benzene may be in industrial effluents discharged into water and accidental releases from gas and oil production, refining and distribution industries. Benzene released to soil will either evaporate very quickly or leach to groundwater. It can be biodegraded by soil and groundwater microbes. Benzene released to surface water should mostly evaporate within a few hours to a few days, depending on quantity, temperature, water turbulence, etc. Although benzene does not degrade by hydrolysis, it may be biodegraded by microbes.
-
貯蔵
work with benzene
should be conducted in a fume hood to prevent exposure by inhalation, and splash
goggles and impermeable gloves should be worn at all times to prevent eye and skin
contact. Benzene should be used only in areas free of ignition sources.
-
輸送方法
UN1114 Benzene, Hazard Class: 3; Labels: 3—
Flammable liquid
-
不和合性
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides, many fluorides and perchlorates,
nitric acid.
-
廃棄物の処理
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed. Dilution with alcohol or acetone to minimize
smoke is recommended. Bacterial degradation is also
possible.
-
法令条例
Current USA and EU legislation does not set maximum limits for benzene in soft drinks. However, the FDA has adopted the Environmental Protection Agency (EPA) maximum contaminant level (MCL) for drinking water of 5 ppb as a quality standard for bottled water. ThisMCL has been used to evaluate the significance of benzene contamination in the soft drinks tested in surveys. The FSA has used the World Health Organization (WHO) guideline level for benzene in water of 10 mg kg-1 as a point of reference for its own survey results.