Aminoglycosides drugs
Aminoglycoside mainly takes effect on the bacterial ribosome in vivo, inhibiting bacterial protein synthesis as well as undermining the integrity of the bacterial cell membrane. Aminoglycoside, through relying on the energy dependent transport system of Ⅱ in vivo, participates into binding to the 30S ribosomal subunit, causing insertion of erroneous proteins into the cell membrane, resulting in the changes in the cell membrane permeability as well as the leakage of intracellular potassium, adenine nucleoside as well as important substances, further resulting in rapid bacterial cell death. The entering of large amount of aminoglycoside molecule into the bacterial cell is an aerobic energy-consuming process. This process will be inhibits under hypoxic conditions. In present time, the clinical applied aminoglycosides all can effectively inhibit the prokaryotic protein synthesis upon reaching the therapeutic concentrations (≤25 μg/ml). Aminoglycosides have strong bactericidal effect against bacteria in stationary phase, being an stationary-phase bactericidal agents.
1. Antibacterial spectrum and drugs resistance; aminoglycoside has strong antibacterial effect against aerobic gram-negative bacteria such as Escherichia coli, Klebsiella, Enterobacter, Proteus, Shigella, Serratia spp., Salmonella spp. However, its antibacterial effect against Alcaligenes, Moraxella, Citrobacter, Acinetobacter, Brucella, meningococcus and other gram-negative bacteria is poor. It also has poor effect against each group of streptococci (such as group-A streptococci, Streptococcus viridans, and Streptococcus pneumoniae). The majority of Enterococcus is resistant to it. Mycobacterium tuberculosis is sensitive to streptomycin. Aminoglycoside has stronger antibacterial effect in an alkaline environment with Ca2 +, Mg2 +, Na +, NH4 +, K + and other cations being capable of inhibiting the antibacterial activity.
2. The in vivo distribution of aminoglycosides; aminoglycoside has poor absorption upon oral administration; only through intestinal infections can it be distributed to a number of vital organs in the body such as penetrating into the pleural or peritoneal effusions. This class of drugs is primarily subject to renal excretion with relatively high urine concentrations, thus being conducive to the treatment of urinary tract infection. However, they have lower concentration in the bile with poor efficacy in treating the biliary tract infections. They are not easy to penetrate through the blood - brain barrier, and thus not suitable to the central infection.
3. Indications; at present time, aminoglycosides are still commonly used drugs in the domestic clinical practice. It is mainly used for the treatment of severe systemic infections caused by aerobic gram-negative bacilli including biliary tract infections, bone and joint infections, pneumonia, sepsis, urinary tract infection, skin and soft tissue infections. However, in case of treatment of serious infection or sepsis caused unknown pathogens, or severe Gram-negative bacilli sepsis, pneumonia, meningitis, or Staphylococcus aureus or Enterococcus infection, this class of drugs is often combined with other antibiotics. Aminoglycoside has a poor antibacterial effect against Streptococcus while the streptococcus is one of the major strains that caused the upper respiratory tract infection. Therefore, in this case, application of this class of drugs is unreasonable, and can not only delay treatment, but also increase the incidence of adverse reactions.
Medication methods; in case of dealing with serious infections, regardless of whether the patients have normal renal function or not, they should be subject to the first-time impulse amount in order to ensure the achievement of effective concentration in the tissues. The applied dose should be calculated according to the weight with fat subtracted (or standard weight) + 40% × overweight part, since different patients often have greatly varied plasma concentration and half-life, the blood concentration should be monitored in conditions allowed to adjust the dose so that individualized dosing can be achieved.
4. The principle of combination therapy; aminoglycoside, when used in combination with penicillins or cephalosporin, can often lead to synergies effects. The combinations of penicillin and streptomycin have synergistic effect against Streptococcus viridans. Other possible combinations with potential synergistic effect include: the combination with enzyme-resistant semi-synthetic penicillin (such as oxacillin) for treatment of Staphylococcus aureus; the combination with penicillin (or ampicillin) or vancomycin for treatment of Enterococcus; the combination with cephalosporin for the treatment of Klebsiella pneumoniae; the combination with penicillin or ampicillin for the treatment of Listeria; the combination with piperacillin and carbenicillin for the treatment of Pseudomonas aeruginosa.
Precautions
1. Aminoglycosides have cross-allergy with each other with allergic patients disabled.
2. Combination with penicillin G can have synergistic antibacterial effect against almost all kinds of Streptococcus faecalis and its variants such as Streptococcus faecium species and Streptococcus durans. Combination with enough amount of carbenicillin has synergistic antibacterial effect against some sensitive strains of Pseudomonas aeruginosa.
3. Combination with alkaline drugs (such as sodium bicarbonate, aminophylline, etc.) can enhance the antibacterial effectiveness but can also lead to a corresponding increase in the toxicity; combination with strong diuretics (such as furosemide, ethacrynic acid, etc.) can increase the renal toxicity; combination with other ototoxic drugs (such as erythromycin, etc.) can increase the ototoxicity; combination with cephalosporins can increase the renal toxicity.
- Structure:
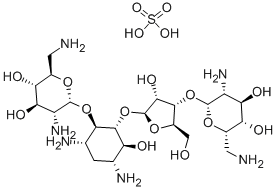
- Chemical Name:Neomycin sulfate
- CAS:1405-10-3
- MF:C23H48N6O17S
- Structure:
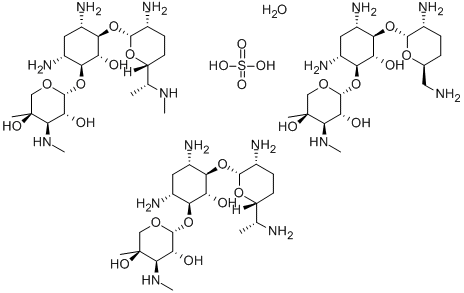
- Chemical Name:Gentamicin sulfate
- CAS:1405-41-0
- MF:C60H127N15O26S
- Structure:
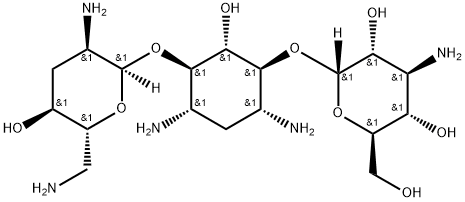
- Chemical Name:Tobramycin
- CAS:32986-56-4
- MF:C18H37N5O9
- Structure:
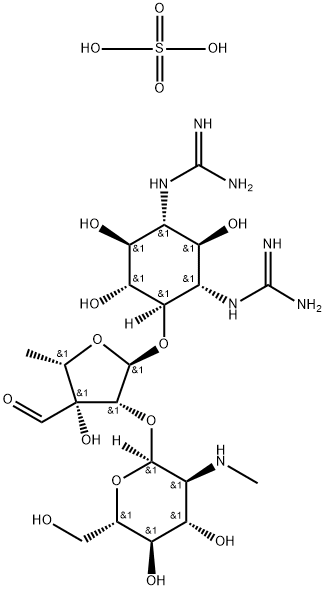
- Chemical Name:Streptomycin sulfate
- CAS:3810-74-0
- MF:C21H41N7O16S
- Structure:
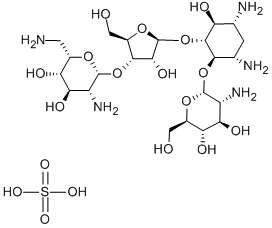
- Chemical Name:PAROMOMYCIN SULFATE
- CAS:1263-89-4
- MF:C23H47N5O18S
- Structure:
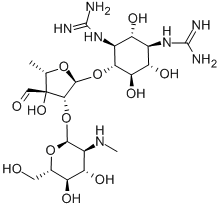
- Chemical Name:Streptomycin
- CAS:57-92-1
- MF:C21H39N7O12
- Structure:
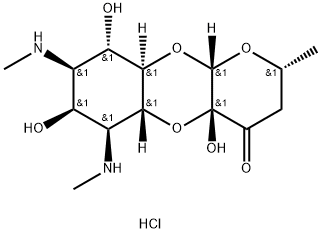
- Chemical Name:SPECTINOMYCIN DIHYDROCHLORIDE
- CAS:21736-83-4
- MF:C14H25ClN2O7
- Structure:
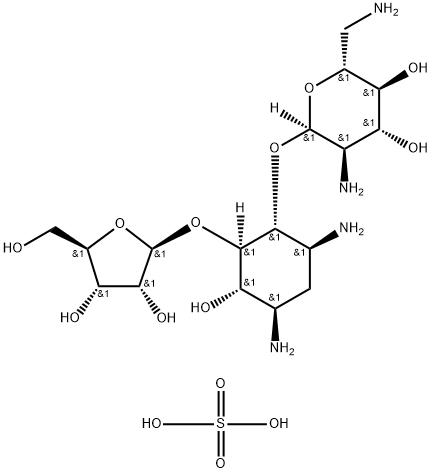
- Chemical Name:Ribostamycin sulfate
- CAS:53797-35-6
- MF:C17H36N4O14S
- Structure:
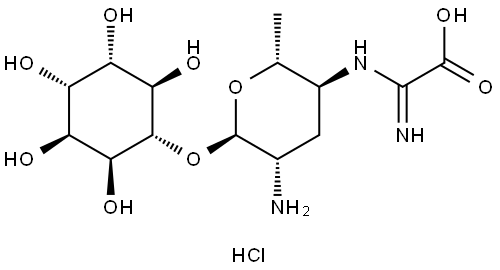
- Chemical Name:Kasugamycin hydrochloride
- CAS:19408-46-9
- MF:C14H25N3O9.ClH
- Structure:
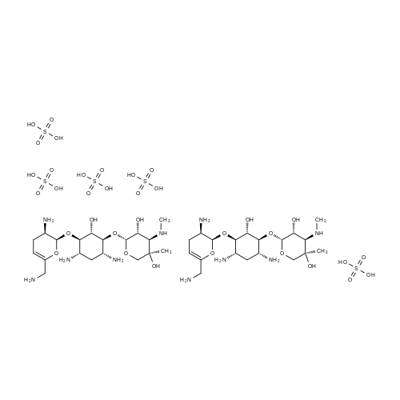
- Chemical Name:Sisomycin Sulfate
- CAS:53179-09-2
- MF:C19H39N5O11S
- Structure:
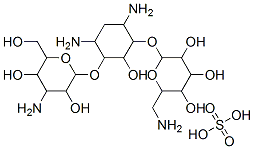
- Chemical Name:KANAMYCIN SULFATE
- CAS:70560-51-9
- MF:C18H38N4O15S
- Structure:
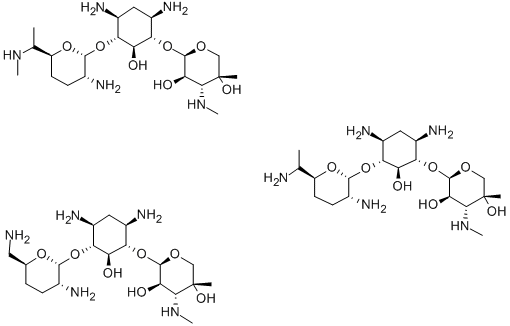
- Chemical Name:Gentamicin
- CAS:1403-66-3
- MF:C60H123N15O21
- Structure:
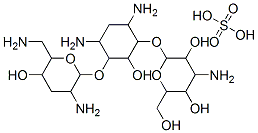
- Chemical Name:Tobramycin sulfate
- CAS:79645-27-5
- MF:C18H39N5O13S
- Structure:
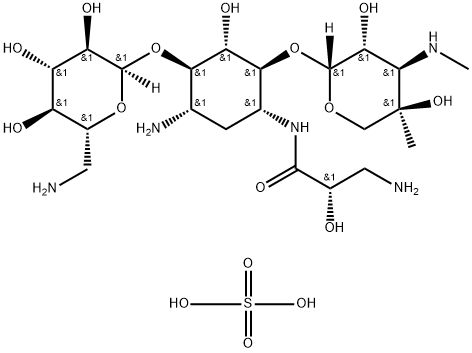
- Chemical Name:Isepamicin sulfate
- CAS:67814-76-0
- MF:C22H45N5O16S
- Structure:
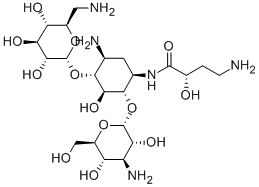
- Chemical Name:AMIKACIN
- CAS:37517-28-5
- MF:C22H43N5O13
- Structure:
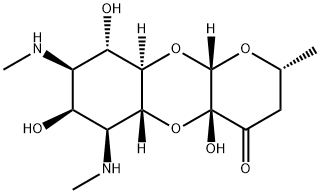
- Chemical Name:Spectinomycin
- CAS:1695-77-8
- MF:C14H24N2O7
- Structure:
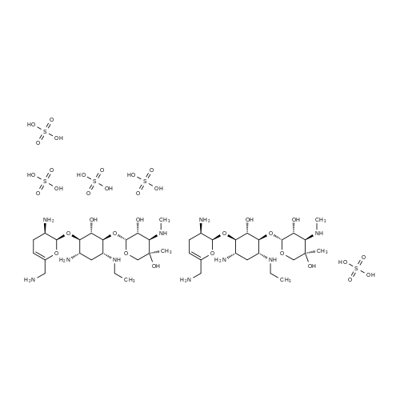
- Chemical Name:Netilmicin sulfate
- CAS:56391-57-2
- MF:C42H92N10O34S5
- Structure:
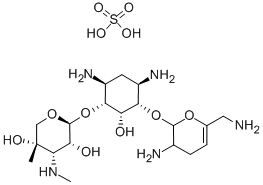
- Chemical Name:Sisomicin
- CAS:32385-11-8
- MF:C19H37N5O7
- Structure:
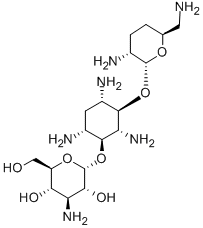
- Chemical Name:(2R,3R,4S,5S,6R)-4-Amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-(aminomethyl)oxan-2-yl]oxy-2-hydroxy-cyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol
- CAS:34493-98-6
- MF:C18H37N5O8
- Structure:
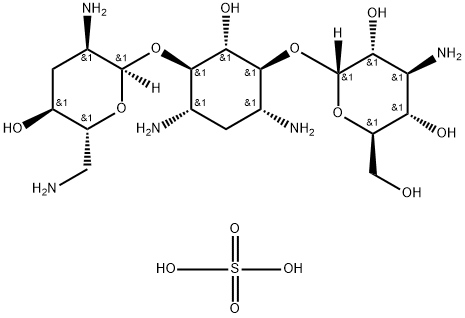
- Chemical Name:Tobramycin sulfate
- CAS:49842-07-1
- MF:C18H39N5O13S
- Structure:
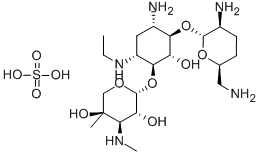
- Chemical Name:Etimicin Sulphate
- CAS:362045-44-1
- MF:C21H43N5O7.H2O4S
- Structure:
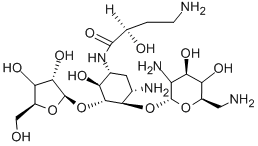
- Chemical Name:Butirosin
- CAS:12772-35-9
- MF:C21H41N5O12
- Structure:
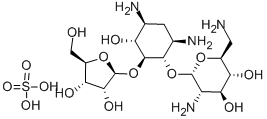
- Chemical Name:RIBOSTAMYCIN SULFATE SALT
- CAS:25546-65-0
- MF:C17H34N4O10
- Structure:
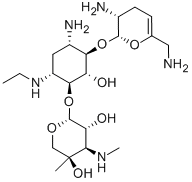
- Chemical Name:NETILMICIN
- CAS:56391-56-1
- MF:C21H41N5O7
- Structure:
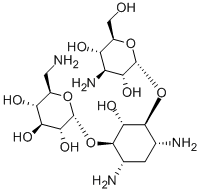
- Chemical Name:KANAMYCIN
- CAS:59-01-8
- MF:C18H36N4O11
- Chemical Name:Framycetine sulphate BP98
- CAS:
- MF:
- Structure:
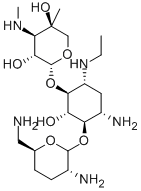
- Chemical Name:Etimicin
- CAS:59711-96-5
- MF:C21H43N5O7
- Structure:
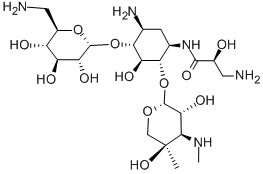
- Chemical Name:Isepamicine
- CAS:58152-03-7
- MF:C22H43N5O12
- Structure:
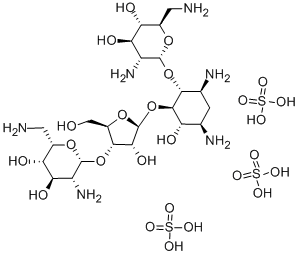
- Chemical Name:Neomycin
- CAS:1404-04-2
- MF:C23H52N6O25S3
- Chemical Name:Viomycin sulfzte
- CAS:
- MF:C25H45N13O14S
- Chemical Name:Nebramycin
- CAS:11048-13-8
- MF:
- Structure:
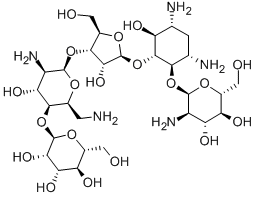
- Chemical Name:Lividomycin
- CAS:36019-37-1
- MF:C29H55N5O19
- Structure:
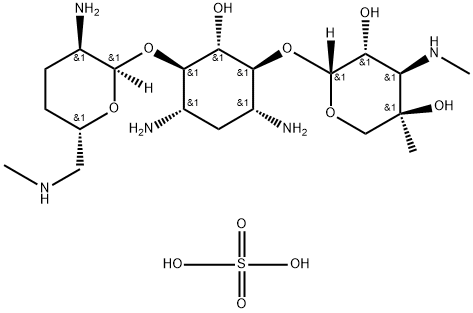
- Chemical Name:MICRONOMICIN SULFATE
- CAS:66803-19-8
- MF:C20H43N5O11S
- Structure:
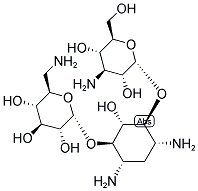
- Chemical Name:KANAMYCIN
- CAS:8063-07-8
- MF:C18H36N4O11
- Structure:
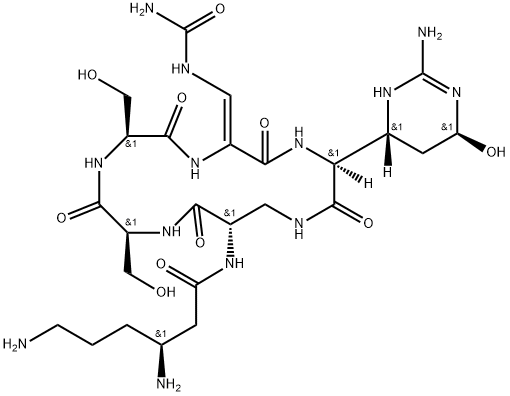
- Chemical Name:viomycin
- CAS:32988-50-4
- MF:C25H43N13O10
- Structure:
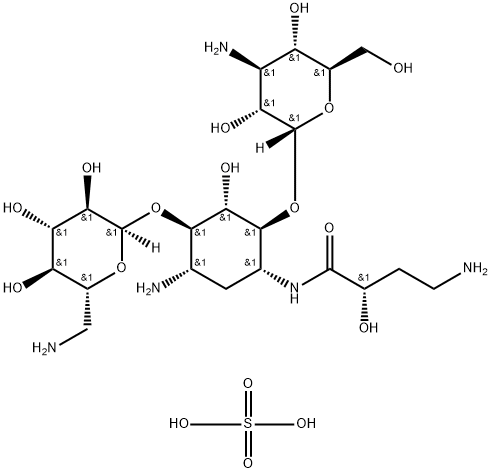
- Chemical Name:Amikacin sulfate salt
- CAS:149022-22-0
- MF:C22H45N5O17S
- Structure:
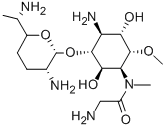
- Chemical Name:FORTIMICIN
- CAS:55779-06-1
- MF:C17H35N5O6
- Structure:
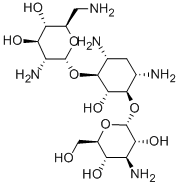
- Chemical Name:Bekanamycin
- CAS:4696-76-8
- MF:C18H37N5O10
- Structure:
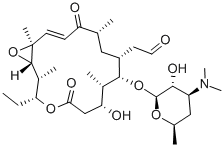
- Chemical Name:ROSAMICIN
- CAS:35834-26-5
- MF:C31H51NO9
- Chemical Name:KANAMYCIN MONOSULPHATE
- CAS:
- MF:C18H38N4O15S
- Structure:
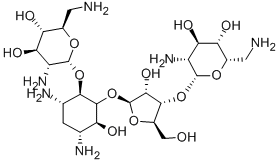
- Chemical Name:NEOMYCIN B
- CAS:119-04-0
- MF:C23H46N6O13
- Structure:

- Chemical Name:Dibekacin sulfate
- CAS:58580-55-5
- MF:C18H37N5O11S
- Structure:
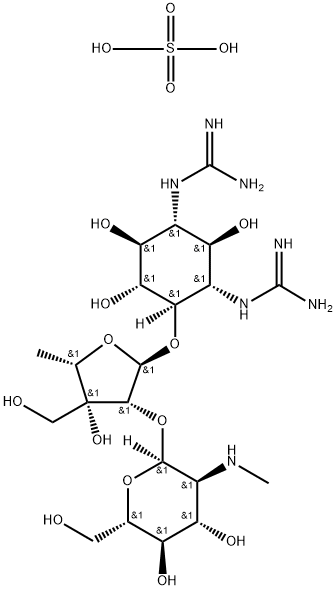
- Chemical Name:DIHYDROSTREPTOMYCIN SESQUISULFATE SALT
- CAS:1425-61-2
- MF:C21H43N7O16S
- Structure:
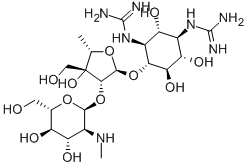
- Chemical Name:Dihydrostreptomycin
- CAS:128-46-1
- MF:C21H41N7O12