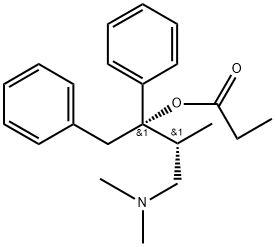
alpha-(2-(Dimethylamino)-1-methyl-ethyl)-alpha-phenylbenzolethanol-propanoat, (S-(R*,S*))-
Bezeichnung:alpha-(2-(Dimethylamino)-1-methyl-ethyl)-alpha-phenylbenzolethanol-propanoat, (S-(R*,S*))-
CAS-Nr469-62-5
Englisch Name:PROPOXYPHENE
CBNumberCB4716127
SummenformelC22H29NO2
Molgewicht339.48
MOL-Datei469-62-5.mol
Synonyma
Dextropropoxyphen
alpha-(2-(Dimethylamino)-1-methyl-ethyl)-alpha-phenylbenzolethanol-propanoat, (S-(R*,S*))-
alpha-(2-(Dimethylamino)-1-methyl-ethyl)-alpha-phenylbenzolethanol-propanoat, (S-(R*,S*))- physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 75-76° |
| alpha | D25 +67.3° (c = 0.6 in chloroform) |
| Siedepunkt | 475.43°C (rough estimate) |
| Dichte | 1.0751 (rough estimate) |
| Brechungsindex | 1.5614 (estimate) |
| Flammpunkt | 2℃ |
| storage temp. | 2-8°C |
| pka | pKa 6.3(50% aq EtOH) (Uncertain) |
| EPA chemische Informationen | Propoxyphene (469-62-5) |
| Kennzeichnung gefährlicher | F,Xn,T |
| R-Sätze: | 11-20/21/22-36-52/53-25 |
| S-Sätze: | 16-26-36/37-61-45-36/37/39 |
| RIDADR | 3249 |
| WGK Germany | 2 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Giftige Stoffe Daten | 469-62-5(Hazardous Substances Data) |
| Toxizität | An opioid analgesic similar in structure to methadone. It is a much less potent analgesic than morphine and is devoid of antipyretic or anti-inflammatory effects. Its side effects are qualitatively similar to codeine. The clinical indications for propoxyphene are much the same as for aspirin. It is used when the degree of analgesia required is less than that produced by morphine. Recent clinical trials suggest that propoxyphene is no more effective in controlling mild pain than is aspirin. Because they act by different mechanisms, aspirin and propoxyphene have often been given in combination. Recently, there has been a trend away from use of propoxyphene because its weak analgesic properties do not compensate for other problems with its use. Interestingly, the analgesic activity of propoxyphene resides largely in the dextrorotatory isomer, whereas the antitussive effect is produced primarily by the levorotatory isomer. The use of propoxyphene was banned in the United States in November 2010. The oral LD50 in rats is 84 mg/kg. |


