General Description
A white to dirty gray crystalline solid. Water soluble. Noncombustible, but accelerates the burning of combustible materials. If large quantities are involved in fire or the combustible material is finely divided an explosion may result. May explode under prolonged exposure to heat or fire. Toxic oxides of nitrogen are produced in fires. Used in solid propellants, explosives, fertilizers.
Reactivity Profile
POTASSIUM NITRATE(7757-79-1) mixed with alkyl esters may explode, owing to the formation of alkyl nitrates; mixtures with phosphorus, tin (II) chloride, or other reducing agents may react explosively [Bretherick 1979. p. 108-109]. Powdered antimony mixed with POTASSIUM NITRATE(7757-79-1) explodes when heated [Mellor 9:282 1946-47]. A mixture of antimony trisulfide and POTASSIUM NITRATE(7757-79-1) explodes at a red heat [Mellor 9:524. 1946-47]. Arsenic disulfide forms explosive mixtures when mixed with POTASSIUM NITRATE(7757-79-1), [Mellor 9:270.1946-47]. A mixture of sodium acetate and POTASSIUM NITRATE(7757-79-1) may cause an explosion [Pieters 1957. p. 30]. A mixture of POTASSIUM NITRATE(7757-79-1) and sodium hypophosphite constitutes a powerful explosive [Mellor 8:881. 1946-47]. A mixture of powdered zirconium and POTASSIUM NITRATE(7757-79-1) explodes when heated above the melting point [Mellor 7:116. 1946-47].
Air & Water Reactions
Soluble in water.
Hazard
Dangerous fire and explosion risk when
shocked or heated, or in contact with organic mate-
rials, strong oxidizing agent.
Health Hazard
Exposure can cause mild irritation of eyes, nose and throat.
Potential Exposure
Used to make explosives, gunpowder,
fireworks, rocket fuel; matches, fertilizer, fluxes, glass
manufacture; and as a diuretic
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least 15
minutes, occasionally lifting upper and lower lids. Seek medi-
cal attention immediately. If this chemical contacts the skin,
remove contaminated clothing and wash immediately with
soap and water. Seek medical attention immediately. If this
chemical has been inhaled, remove from exposure, begin res-
cue breathing (using universal precautions, including resusci-
tation mask) if breathing has stopped and CPR if heart action
has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention.
Give large quantities of water and induce vomiting. Do not
make an unconscious person vomit.
Shipping
UN1486 Potassium nitrate, Hazard Class: 5.1;
Labels: 5.1-Oxidizer.
Incompatibilities
A powerful oxidizer. Dangerously
reactive and friction-and shock-sensitive when mixed with
organic materials and many materials. Violent reactions
with reducing agents; chemically active metals; charcoal,
trichloroethylene.
Chemical Properties
Potassium nitrate is an odorless, flammable, water-soluble, white or colorless crystals with saline taste that melt at 337°C. Used in pyrotechnics, explosives, and matches, as a fertilizer, and as an analytical reagent.
Physical properties
Colorless transparent crystals or white granular or crystalline powder;rhombohedral structure; density 2.11 g/cm3at 20°C; melts at 334°C; decomposes at 400°C evolving oxygen; soluble in cold water, 13.3 g/100mL at 0°C;highly soluble in boiling water, 247 g/100mL at 100°C; lowers the temperature of water on dissolution; very slightly soluble in ethanol; soluble in glycerol and liquid ammonia.
History
Saltpeter’s most prominent use in human history is
as the principal ingredient in gunpowder.The potassium nitrate used in gunpowder was originally obtained from natural mineral
deposits of niter. Small quantities formed as efflorescence deposits on damp stone walls were identified as early as 2000 b.c.e. in Sumerian writings. As the use of black powder expanded
with the development of weapons, the demand for saltpeter exceeded supply. This was exacerbated
during times of war. To meet the demand for saltpeter to produce black powder, a saltpeter
industry developed that followed prescribed methods to produce large quantities of saltpeter.
The method depended on processing dirt obtained from areas where nitrates would naturally
form. These were areas in which animal waste had accumulated such as the dirt
floors of barns, stables, herding pens, caves, or cellars. The ammonia compounds in the urine
and fecal wastes in these areas underwent nitrifi cation to produce nitrates, which combined
with potassium in the soil to form saltpeter.
Definition
ChEBI: The inorganic nitrate salt of potassium.
Production Methods
Potassium nitrate may be produced by several methods. It is made commercially by reacting potassium chloride with nitric acid at high temperature.Nitrosyl chloride, a product obtained in the reaction, is converted into chlorine in this manufacturing process. Also, nitric acid is partly recycled in the process. The reactions are (Dancy, W.B. 1981. Potassium Compounds. In Kirk-Othmer Encyclopedia of Chemical Technology, 3rd. ed. Pp. 939-42. New York: Wiley Interscience):
3KCl + 4HNO3 →3KNO3+ Cl2+ NOCl + 2H2O
2NOCl + 4HNO3→6NO2+ Cl2+ 2H2O
4NO2+ O2+ 2H2O →4HNO3
Potassium nitrate also can be prepared by mixing a hot saturated solution of potassium chloride and sodium nitrate. The reaction is:
K++ Clˉ+ Na++ NO3ˉ→NaCl↓+ K++ NO3ˉ
Sodium chloride is less soluble than KCl, NaNo3and KNo3. It separates out by crystallization. The remaining solution is cooled to ambient tempera-ture. Potassium nitrate crystallizes out.
Brand name
Cholal modifico;Cholal simple;Dewitt's pills for backache and joint pain;Viridite k.
World Health Organization (WHO)
Potassium nitrate was formerly used as a diuretic. Its use for this
purpose is now considered obsolete but it is still available in at least one country
for the correction of potassium deficiency. It is aslo widely permitted at
concentrations of the order of 5% in proprietary toothpastes. In some countries the
drug has been banned due to a potential carcinogenic risk arising from the
excessive use of nitrates and their transformation to nitrosamines.
Flammability and Explosibility
Nonflammable
Agricultural Uses
Potassium nitrate (KNO3) is a potassium salt of nitric acid, also known as saltpeter or nitrate of potash. It is a white crystalline salt which occurs naturally in nitre or saltpeter. It can be used as fertilizer for normal application and fertigation. Potassium (44% K2O) and nitrogen (13 %) are the constituents of NK fertilizers, which serve as a source of potassium, where extra chloride is not desired.
The agricultural grade of potassium nitrate is freeflowing and non-caking, with a particle size in the range of 1500 to 400 microns.
Potassium nitrate, which is slightly hygroscopic and granulated, can be spread on soil by trucks, fertilizer distributors or by aerial spraying. In a mixed fertilizer, a powdered grade of nitrate of potash does not cake. Potassium nitrate is made by the reaction of potassium chloride with nitric acid as: The nitrate of potash forms an easily breakable crust on top. It is chemically neutral and its nitrogen and potassium oxide ratio is roughly 1:3. It has been used successfully as a source of nitrogen and potassium for tobacco, tomato, potato, corn, citrus and carnations.
Industrial uses
Potassium nitrate is also called niter and saltpeter,although these usually refer to the nativemineral. A substance of the composition KNO3,it is used in explosives, for bluing steel, and infertilizers. A mixture of potassium nitrate andsodium nitrate is used for steel-tempering baths.The mixture melts at 250°C. Potassium nitrateis made by the action of potassium chloride onsodium nitrate. It occurs in colorless prismaticcrystals, or as a crystalline white powder. It hasa sharp saline taste and is soluble in water. Thespecific gravity is 2.1 and the melting point is337°C.
Potassium nitrate contains a large percentageof oxygen, which is readily given up andis well adapted for pyrotechnic compounds. Itgives a beautiful violet flame in burning. It isused in flares and in signal rockets.
Most enamels contain some oxidizing agentin the form of potassium or sodium nitrate.Only a small amount of nitrate is necessary; 2to 4% is sufficient to maintain oxidizing conditionsin most smelting operations.
In glazes it is sometimes used as a flux inplace of potassium oxide, but, owing to its costand solubility, very little of it is contained inglaze. Where conditions prevent the use of sufficientpotash feldspar, potassium oxide is introducedinto the mix, usually in the form of thenitrate in a frit.
Potassium nitrite is a solid of the compositionKNO2 used as a rust inhibitor, for theregeneration of heat-transfer salts, and for themanufacture of dyes.
Biochem/physiol Actions
Potassium nitrate serves as a source of nitrate for cell growth. It also induces the synthesis of lipid in alga Neochloris oleoabundans.
Purification Methods
It crystallises from hot H2O (0.5mL/g) on cooling (cf KNO2 below). Dry it for 12hours under vacuum at 70o. The solubility in H2O is 13.3% at 0o, 110% at 60o, and 246% at 100o. After two recrystallisations, technical grade salt had <0.001 ppm of metals. The fused salt is a powerful oxidising agent.
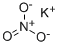
 ;
;