General Description
A pale yellow crystalline solid with a faint odor of rotten eggs. Insoluble in water. A fire and explosion risk above 450° F. Transported as a yellow to red liquid. Handled at elevated temperature (typically 290°F) to prevent solidification and makes transfers easier. Hot enough that plastic or rubber may melt or lose strength. Causes thermal burns to skin on contact. Cools rapidly and solidifies if released. Equipment designed to protect against ordinary chemical exposure is ineffective against the thermal hazard. Exercise caution walking on the surface of a spill to avoid breakthrough into pockets of molten sulfur below the crust. Do not attempt to remove sulfur impregnated clothing because of the danger of tearing flesh if a burn has resulted. May be irritatin to skin, eyes and mucous membranes. Used in sulfuric acid production, petroleum refining, and pulp and paper manufacturing.
Reactivity Profile
SULFUR reacts violently with strong oxidizing agents causing fire and explosion hazards [Handling Chemicals Safely 1980 p. 871]. Reacts with iron to give pyrophoric compounds. Attacks copper, silver and mercury. Reacts with bromine trifluoride, even at 10°C [Mellor 2:113. 1946-47]. Ignites in fluorine gas at ordinary temperatures [Mellor 2:11-13 1946-47]. Reacts to incandescence with heated with thorium [Mellor 7:208 1946-47]. Can react with ammonia to form explosive sulfur nitride. Reacts with calcium phosphide incandescently at about 300°C. Reacts violently with phosphorus trioxide [Chem. Eng. News 27:2144 1949]. Mixtures with ammonium nitrate or with metal powders can be exploded by shock [Kirk and Othmer 8:644]. Combinations of finely divided sulfur with finely divided bromates, chlorates, or iodates of barium, calcium, magnesium, potassium, sodium, or zinc can explode with heat, friction, percussion, and sometimes light [Mellor 2 Supp.1:763. 1956]. A mixture with barium carbide heated to 150°C becomes incandescent. Reacts incandescently with calcium carbide or strontium carbide at 500°C. Attacks heated lithium, or heated selenium carbide with incandescence [Mellor 5:862 1946-47]. Reacts explosively if warmed with powdered zinc [Mellor 4:476. 1946-47]. Reacts vigorously with tin [Mellor 7:328. 1946-47]. A mixture with potassium nitrate and arsenic trisulfide is a known pyrotechnic formulation [Ellern 1968 p. 135]. Mixtures with any perchlorate can explode on impact [ACS 146:211-212]. A mixture of damp sulfur and calcium hypochlorite produces a brilliant crimson flash with scatter of molten sulfur [Chem. Eng. News 46(28):9 1968]. Takes fire spontaneously in chlorine dioxide and may produce an explosion [Mellor 2:289 (1946-47)]. Ignites if heated with chromic anhydride ignite and can explode, [Mellor 10:102 (1946-47)]. Even small percentages of hydrocarbons in contact with molten sulfur generate hydrogen sulfide and carbon disulfide, which may accumulate in explosive concentrations. Sulfur reacts with Group I metal nitrides to form flammable mixtures, evolving flammable and toxic NH3 and H2S gasses if water is present. (Mellor, 1940, Vol. 8, 99).
Air & Water Reactions
Flammable. Insoluble in water.
Health Hazard
Can cause eye irritation; may rarely irritate skin. If recovered sulfur, refer to hydrogen sulfide.*
Potential Exposure
Widely used in manufacture of sulfuric acid; carbon bisulfide; drugs, fungicides, gunpowder, wood pulp; rubber, and other products.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Shipping
UN1350 Sulfur, Hazard Class: 4.1; Labels: 4.1-Flammable solid (International). NA1350 Sulfur, Hazard class: 9; Labels: 9-Miscellaneous hazardous material (Domestic). UN2448 Sulfur, molten, Hazard Class: 4.1; Labels: 4.1-Flammable solid (International). NA 2448 Sulfur, molten Hazard class: 9; Labels: 9-Miscellaneous hazardous material (Domestic).
Incompatibilities
Widely used in manufacture of sulfuric acid; carbon bisulfide; drugs, fungicides, gunpowder, wood pulp; rubber, and other products.
Chemical Properties
Sulfur is a yellow crystalline solid or powder. Often transported in the molten state.
Chemical Properties
Sulfur, S, is a nonmetallic element that exists in a crystalline or amorphous form and in four stable isotopes. Sulfur melts at temperatures rangingfrom 112.8°C (234 °F) for the rhombic form to 120.0°C(248 °F) for amorphous sulfur,and all forms boil at 444.7°C (835°F). Sulfur occurs as free sulfur in many volcanic areas and is often associated with gypsum and limestone. It is used as a chemical intermediate and fungicide and in the vulcanization of rubber.
Waste Disposal
Salvage for reprocessing or dump to landfill.
Physical properties
Sulfur is considered a nonmetallic solid. It is found in three allotropic crystal forms:
1. Orthorhombic (or rhombic) octahedral lemon-yellow crystals, which are also called“brimstone” and referred to as “alpha” sulfur. The density of this form of sulfur is 2.06g/cm3, with a melting point of 95.5°C.
2. Monoclinic, prismatic crystals, which are light-yellow in color. This allotrope is referredto as “beta” sulfur. Its density is 1.96 g/cm3, with a melting point of 119.3°C.
3. Amorphous sulfur is formed when molten sulfur is quickly cooled. Amorphous sulfur issoft and elastic, and as it cools, it reverts back to the orthorhombic allotropic form.
Sulfur, in its elemental form, is rather common and does not have a taste or odor except whenin contact with oxygen, when it forms small amounts of sulfur dioxide.
Isotopes
There are a total of 24 isotopes of sulfur; all but four of these are radioactive.The four stable isotopes and their contribution to sulfur’s total abundance on Earth areas follows: S-32 contributes 95.02% to the abundance of sulfur; S-33, just 0.75%; S-34,4.21%; and S-36, 0.02%.
Origin of Name
From the Sanskrit word sulvere and the Latin word sulphurim.
Occurrence
Sulfur has been known since ancient times primarily because it is a rather common substance.It is the 15th most common element in the universe, and though it is not found in allregions of the Earth, there are significant deposits in south Texas and Louisiana, as well in allvolcanoes. Sulfur makes up about 1% of the Earth’s crust.
Sulfur is an element found in many common minerals, such as galena (PbS), pyrite(fool’s gold, FeS2), sphalerite (ZnS), cinnabar (HgS), and celestite (SrSO4), among others.About 1/4 of all sulfur procured today is recovered from petroleum production. Themajority of sulfur is the result of or a by-product of mining other minerals from the orescontaining sulfur.
Sulfur is mined by the recovery method known as the Frasch process, which was inventedby Herman Frasch in Germany in the early 1900s. This process forces superheated water,under pressure, into deep underground sulfur deposits. Compressed air then forces the moltensulfur to the surface, where it is cooled. There are other methods for mining sulfur, but theFrasch process is the most important and most economical.
Sulfur is found in Sicily, Canada, Central Europe, and the Arabian oil states, as well as inthe southern United States in Texas and Louisiana and offshore beneath the Gulf of Mexico.
Characteristics
Sulfur exhibits a remarkable array of unique characteristics. Today, there are chemistsdevoting large portions of their careers to studying this unusual element. For example, whensulfur is melted, its viscosity increases, and it turns reddish-black as it is heated. Beyond200°C, the color begins to lighten, and it flows as a thinner liquid.
Sulfur burns with a beautiful subdued blue flame. The old English name for sulfur was“brimstone,” which means “a stone that burns.” This is the origin of the term “fire and brimstone”when referring to great heat. Above 445°C, sulfur turns to a gas, which is dark orangeyellowbut which becomes lighter in color as the temperature rises.
Sulfur is an oxidizing agent and has the ability to combine with most other elements toform compounds.
Definition
sulphur: Symbol S. A yellow nonmetallic element belonging to group 16 (formerly VIB) of the periodic table; a.n. 16; r.a.m. 32.06; r.d. 2.07 (rhombic); m.p. 112.8°C; b.p. 444.674°C. The element occurs in many sulphide and sulphate minerals and native sulphur is also found in Sicily and the USA (obtained by the Frasch process). It can also be obtained from hydrogen sulphide by the Claus process.
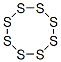
Sulphur has various allotropic forms. Below 95.6°C the stable crystal form is rhombic; above this temperature the element transforms into a triclinic form. These crystalline forms both contain cyclic S8 molecules. At temperatures just above its melting point, molten sulphur is a yellow liquid containing S8 rings (as in the solid form). At about 160°C,the sulphur atoms form chains and the liquid becomes more viscous and dark brown. If the molten sulphur is cooled quickly from this temperature (e.g. by pouring into cold water) a reddish-brown solid known as plastic sulphur is obtained. Above 200°C the viscosity decreases. Sulphur vapour contains a mixture of S2, S4, S6, and S8 molecules. Flowers of sulphur is a yellow powder obtained by subliming the vapour. It is used as a plant fungicide. The element is also used to produce sulphuric acid and other sulphur compounds.
Sulphur is an essential element in living organisms, occurring in the amino acids cysteine and methionine and therefore in many proteins. It is also a constituent of various cell metabolites, e.g. coenzyme A. Sulphur is absorbed by plants from the soil as the sulphate ion (SO42–).
Brand name
Liquamat (Galderma ); Sastid (Stiefel); Sulfur Soap (Stiefel).
Hazard
Many of the sulfur compounds are toxic but essential for life. The gas from elemental sulfurand from most of the compounds of sulfur is poisonous when inhaled and deadly wheningested. This is the reason that sulfur compounds are effective for rat and mice exterminationas well an ingredient of insecticides. Sulfa drugs (sulfanilamide and sufadiazine), althoughtoxic, were used as medical antibiotics during World War II before the development of penicillin.They are still used today in veterinary medicine.
Flammability and Explosibility
Nonflammable
Agricultural Uses
Brimstone is coarsely ground sulphur which is used to
increase the acidity of soil and correct sulphur deficiency
inplants.
Agricultural Uses
Sulphur (S) is a yellow, non-metallic element belonging
to Group 16 (formerly VI B) of the Periodic Table. It is a macronutrient required by plants in
relatively large amounts. Potato, cereals and grasses
require about 20 kg/ha sulphur, while its ideal dosage for
the Brassiceae family of crops is 50 kg/ha.
Soil-sulphur reactions are similar to soil-nitrogen
reactions, and are dominated by organic or microbial
fractions in the soil. Approximately 90% sulphur
required by plants is for the synthesis of amino acids
(namely, cysteine, cystine and methionhe) which are essential components of proteins and contain 6 to 8% S.
One of the main functions of sulphur in proteins is to form
sulphur-sulphur (-S-S-) bonds between polypeptide
chains which are essential for the protein conformation
relevant to its catalytic or structural properties.
Depending on their sulphur requirement, crops are
divided into three groups. The first includes crops with a
high sulphur requirement - in a range 20 to 80 kg/ha.
Crucifers and Brassiceae fall in this group. The second
group requires sulphur in a moderate range - 10 to 50
kg/ha and includes plantation crops. The third group
needs sulphur in small quantities - 5 to 25 kg/ha and
includes cereals, forages and other field crops. As a rule,
sulphur requirement is 3 to 4 kg/ton of grains, 8 kg/ton of
grain legumes and 12 kg/ton of oil seeds.
Soil can lose sulphur by (a) its removal by crops,
(b) leaching and erosion, (c) sulphate adsorption
and retention by clays, and (d) cultivation.
Decomposition of organic matter is accelerated by
cultivation, which improves soil segregation and
aeration. The oxidation of organic matter causes a
decline in organic sulphur.
Sulphur is needed for the synthesis of co-enzyme A,
biotin, thiamine and glutathione. It is also present in
substances like sulphur-adenosyl methionhe, formyl
methionhe, lipoic acid and sulfolipid.
Sulphur plays an important role in chlorophyll
synthesis. It is part of ferridoxins, a type of non-heme
iron-sulphur (Fe-S) protein occurring in chloroplasts and
involved in the reduction of nitrite and sulphate, and in
the assimilation of nitrogen by bacteria.
Sulphur enhances the formation of oil in crops like
soybean and flax. Plant roots absorb sulphur as sulphate
ions. Small quantities of sulphur dioxide (SO2) can be
absorbed through plant leaves and used in the plant. The
concentration of sulphur in plants is 0.1 to 4% which is
equal to, or less than, the amount of phosphorus in wheat,
corn, beans and potato but is more than the phosphorus
content in alfalfa, cabbage and turnip.
There is a close relationship between organic C, total
N and total S in soils. The C: N: S ratio in most welldrained,
non-calcareous soils is approximately
120: 10: 1.4. Generally, the C: S ratio varies much more
than the N: S ratio, the latter falling within a narrow
range of 6 to 8.1. Sulphur may be immobilized in soils
whenthe C: S or N: S ratio is large.
Purification Methods
Murphy, Clabaugh & Gilchrist [J Res Nat Bur Stand 64A 355 1960] have obtained sulfur of about 99.999% purity by the following procedure: Roll sulfur was melted and filtered through a coarse-porosity glass filter funnel into a 2L round-bottomed Pyrex flask with two necks. Conc H2SO4 (300mL) was added to the sulfur (2.5kg), and the mixture was heated to 150o, stirring continuously for 2hours. Over the next 6hours, conc HNO3 was added in about 2mL portions at 10-15minutes intervals to the heated mixture. It was then allowed to cool to room temperature and the acid was poured off. The sulfur was rinsed several times with distilled water, then remelted, cooled, and rinsed several times with distilled water again, this process being repeated four or five times to remove most of the acid entrapped in the sulfur. An air-cooled reflux tube (ca 40cm long) was attached to one of the necks of the flask, and a gas delivery tube (the lower end about 2.5cm above the bottom of the flask) was inserted into the other. While the sulfur was boiled under reflux, a stream of helium or N2 was passed through to remove any water, HNO3 or H2SO4, as vapours. After 4hours, the sulfur was cooled so that the reflux tube could be replaced by a bent air-cooled condenser. The sulfur was then distilled, rejecting the first and the final 100mL portions, and transferred in 200mL portions to 400mL glass cylinder ampoules (which were placed on their sides during solidification). After adding about 80mL of water, displacing the air with N2, the ampoule was cooled, and the water was titrated with 0.02M NaOH, the process being repeated until the acid content was negligible. Finally, entrapped water was removed by alternate evacuation to 10mm Hg and refilling with N2 while the sulfur was kept molten. The ampoules were then sealed. Other purifications include crystallisation from CS2 (which is less satisfactory because the sulfur retains appreciable amounts of organic material), *benzene or *benzene/acetone, followed by melting and degassing. It has also been boiled with 1% MgO, then decanted, and dried under a vacuum at 40o for 2days over P2O5. [For the purification of S6, “recrystallised S8” and “Bacon-Fanelli sulfur” see Bartlett et al. J Am Chem Soc 83 103, 109 1961.]