Chemical Properties
metal foil, chunks or powder. The powder of gadolinium is highly flammable; incompatible with strong oxidising agents, halogens, acids; and reacts with water or moisture.
Physical properties
Gadolinium is silvery-white, soft, malleable, and ductile with a metallic luster. It is the secondof what is referred to as the dysprosium, subgroup in the middle of the lanthanide seriesof rare-earths. It tarnishes in air, forming the oxide (Gd2O3) on the surface, which flakes offthe surface, exposing a fresh metal that in turn oxidizes.
Its melting point is 1,313°C, its boiling point is 3,273°C, and its density is 7.90g/cm3.
Isotopes
There are 39 isotopes of gadolinium. Seven of these are stable. They are: Gd-54, which makes up 2.18% of all the gadolinium found in the Earth’s crust; Gd-55,supplying 14.80%; Gd-156, making up 20.47%; Gd-157, constituting 15.56%; and Gd-158, accounting for 24.85%. In addition, there are two isotopes of gadolinium that areradioactive and with such long half-lives that they still exist in the Earth’s crust. They areregarded as stable isotopes along with the other seven. They are Gd-152 (1.08×10+14years), which exists in just 0.20% in abundance, and Gd-160 (1.3×10+21 years), foundin 21.86% abundance.
Origin of Name
Named for the mineral gadolinite, which was named for the French
chemist Johann Gadolin.
Occurrence
Gadolinium is the 40th most abundant element on Earth and the sixth most abundant ofthe rare-earths found in the Earth’s crust (6.4 ppm). Like many other rare-earths, gadoliniumis found in monazite river sand in India and Brazil and the beach sand of Florida as well asin bastnasite ores in southern California. Similar to other rare-earths, gadolinium is recoveredfrom its minerals by the ion-exchange process. It is also produced by nuclear fission in atomicreactors designed to produce electricity.
Characteristics
Gadolinium, unlike most of the rare earths in the dysprosium subgroup, reacts slowlywith water, releasing hydrogen. It is strongly magnetic at low temperatures. Two of its stableisotopes (Gd-155 and Gd-157) have the greatest ability of all natural elements to absorb thermalneutrons to control the fission chain reaction in nuclear reactors. However, few of theseisotopes are found in the ores of gadolinium.
Hazard
The halogens of gadolinium are very toxic, and gadolinium nitrate is explosive. As withmost rare-earths, care should be taken not to inhale fumes or ingest particles of gadolinium.
Flammability and Explosibility
Flammable
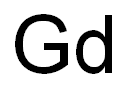
 ;
;