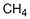Description
All our SWNTs come packed as dry powders, which can be dispersed within the user's solvent of choice.
General Description
Black grains that have been treated to improve absorptive ability. May heat spontaneously if not properly cooled after manufacture.
Reactivity Profile
CARBON, ACTIVATED(7440-44-0) is incompatible with very strong oxidizing agents such as fluorine, ammonium perchlorate, bromine pentafluoride, bromine trifluoride, chlorine trifluoride, dichlorine oxide, chlorine trifluoride, potassium peroxide, etc. . Also incompatible with air, metals, unsaturated oils. [Lewis].
Air & Water Reactions
Highly flammable. Dust is explosive when exposed to heat or flame. Freshly prepared material can heat and spontaneously ignite in air. The presence of water assists ignition, as do contaminants such as oils. Insoluble in water.
Hazard
(Powder, natural) Fire risk.
Health Hazard
Fire may produce irritating and/or toxic gases. Contact may cause burns to skin and eyes. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Potential Exposure
Natural graphite is used in foundry
facings, steel making lubricants, refractories, crucibles,
pencil “lead,” paints, pigments, and stove polish. Artificial
graphite may be substituted for these uses with the excep tion of clay crucibles; other types of crucibles may be pro duced from artificial graphite. Additionally, it may be used
as a high temperature lubricant or for electrodes. It is uti lized in the electrical industry in electrodes, brushes, con tacts, and electronic tube rectifier elements; as a constituent
in lubricating oils and greases; to treat friction elements,
such as brake linings; to prevent molds from sticking
together; and in moderators in nuclear reactors. In addition,
concerns have been expressed about synthetic graphite in
fibrous form. Those exposed are involved in production of
graphite fibers from pitch or acrylonitrile fibers and the
manufacture and use of composites of plastics, metals, or
ceramics reinforced with graphite fibers.
Fire Hazard
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, includ ing resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medi cal attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Shipping
UN1362 Carbon, activated, Hazard Class: 4.2;
Labels: 4.2-Spontaneously combustible material, International.
Incompatibilities
Graphite is a strong reducing agent and
reacts violently with oxidizers, such as fluorine, chlorine
trifluoride, and potassium peroxide. Forms an explosive
mixture with air. May be spontaneously combustible in air.
Chemical Properties
Carbon, C, is a nonmetallic element, grey solid. It is found in nature as graphite (specific gravity2.25), diamond(specific gravity 3.51), and coal (specific gravity 1.88). Carbon is found in all living things, is insoluble in common solvents,and forms an almost infinite numberof organic compounds. Anaturally occurring radioactive isotope,14C, has a half-life of 5780 years and is used in archaeo logical investigations to date artifacts and ancient documents. Other uses of carbon depend on its form. For example, diamonds for jewels and abrasives,graphite for lubricants, activated carbon to absorb color and gases, and wood carbon for fuel are some common examples.
Chemical Properties
Graphite is crystallized carbon and usually
appears as soft, black scales. There are two types of graph ite, natural and artificial (activated). Natural and synthetic
graphite may be mixed with each other or contain other
additives.
Waste Disposal
Do not incinerate. Carbon
(graphite) fibers are difficult to dispose of by incineration.
Waste fibers should be packaged and disposed of in a land fill authorized for the disposal of special wastes of this
nature, or as otherwise may be required by law.
Physical properties
All the elements in group 14 have four electrons in their outer valence shell. Carbon exhibitsmore nonmetallic properties than do the others in group 14 and is unique in several ways.It has four forms, called allotropes:
1. Carbon black is the amorphous allotrope (noncrystal form) of carbon. It is produced byheating coal at high temperatures (producing coke); burning natural gas (producing jetblack); or burning vegetable or animal matter (such as wood and bone), at high temperatureswith insufficient oxygen, which prevents complete combustion of the material, thusproducing charcoal.
2. Graphite is a unique crystal structure of carbon wherein layers of carbon atoms are stackedparallel to each other and can extend indefinitely in two dimensions as in the shafts ofcarbon fiber golf clubs. Graphite is also one of the softest elements, making it an excellentdry lubricant.
3. Diamonds are another allotrope whose crystal structure is similar to graphite. Naturaldiamonds were formed under higher pressure and extreme temperatures. Synthetic diamondshave been artificially produced since 1955.
4. Fullerenes are another amorphous (no crystal structure) form of carbon that have the basicformula of C60H60 and are shaped like a soccer ball. (See the “Atomic Structure” sectionof the book for more on fullerenes.)
The different allotropes of carbon were formed under varying conditions in the Earth,starting with different minerals, temperature, pressure, and periods of time. Once the distinctcrystal structures are formed, they are nearly impossible to change.
Carbon-12 is the basis for the average atomic mass units (amu) that is used to determinethe atomic weights of the elements. Carbon is one of the few elements that can form covalentbonds with itself as well as with many metals and nonmetals.
Isotopes
There are 15 isotopes of carbon, two of which are stable. Stable carbon-12makes up 98.89% of the element’s natural abundance in the Earth’s crust, and carbon-13 makes up just 1.11% of carbon’s abundance in the Earth’s crust. All the otherisotopes of carbon are radioactive with half-lives varying from 30 nanoseconds (C-21) to5,730 years (C-14).
Origin of Name
Carbon’s name is derived from the Latin word carbo, which means,
“charcoal.”
Occurrence
Carbon is the 14th most abundant element, making up about 0.048% of the Earth’s crust.It is the sixth most abundant element in the universe, which contains 3.5 atoms of carbonfor every atom of silicon. Carbon is a product of the cosmic nuclear process called fusion,through which helium nuclei are “burned” and fused together to form carbon atoms withthe atomic number 12. Only five elements are more abundant in the universe than carbon:hydrogen, helium, oxygen, neon, and nitrogen.
Characteristics
Carbon is, without a doubt, one of the most important elements on Earth. It is the majorelement found in over one million organic compounds and is the minor component in mineralssuch as carbonates of magnesium and calcium (e.g., limestone, marble, and dolomite),coral, and shells of oysters and clams.The carbon cycle, one of the most essential of all biological processes, involves the chemicalconversion of carbon dioxide to carbohydrates in green plants by photosynthesis.
Animalsconsume the carbohydrates and, through the metabolic process, reconvert the carbohydratesback into carbon dioxide, which is returned to the atmosphere to continue the cycle.
Agricultural Uses
Carbon (C) is found in every living being as it forms the
major constituent of living cells. As an essential element
for plants and animals, carbon is derived from
atmospheric carbon dioxide assimilated by plants and
photoautotrophic microbes during photosynthesis.
Carbon occurs in nature both in an elemental form and as
compounds. For example, coal contains elemental
carbon which, upon heating in the absence of air, loses
the volatile substances, and gives coke. Both coal and
coke are amorphous (non-crystalline) forms of carbon.
The two crystalline forms of carbon are diamond and
graphite. These are called the two allotropes of carbon.
Allotropes are two or more forms of an element that exist
in different physical forms, and differ in the bonding or
molecular structure of their fundamental units. Carbon is
found in a combined state in all living organisms, as well
as in fossil fuels such as methane and petroleum. It also
occurs in large amounts in carbonates such as limestone.
Carbon, a non-metallic element, is found at the head
of Group 14 (formerly IV) in the Periodic Table. It is unique in the variety and complexity of
compounds it forms, which is due to the ability of carbon
atoms to bond to one another in long chains, rings and
combinations of rings and chains. Carbon in combination
with H, O, N, S and other elements produces such a
variety of compounds, that a separate branch of
chemistry called organic chemistry, came into being
around carbon compounds.
Elemental carbon is a fairly inert substance. It is
insoluble in water, dilute acids and bases, and organic
solvents.
Each carbon atom has four valence electrons and
these tend to share with other atoms in the formation of
four covalent bonds. Carbon forms two oxides - carbon
monoxide (CO) and carbon dioxide (CO2)-which are
formed when carbon or carbon-containing compounds
are burned in insufficient or inexcess air, respectively.
The free element has many uses, ranging from
ornamental applications as diamond in jewelry to the
black-colored pigment of carbon black in automobile
tires and printing inks. Graphite, another form of carbon,
is used for high temperature crucibles, arc lights, dry-cell
electrodes, lead pencils and as a lubricant.
Charcoal, an amorphous form of carbon, is used as
an absorbent for gases and as a decolorizing agent in its
activated form.
Purification Methods
Charcoal (50g) is added to 1L of 6M HCl and boiled for 45minutes. The supernatant is discarded, and the charcoal is boiled with two more lots of HCl, then with distilled water until the supernatant no longer gives a test for chloride ion. The charcoal (now phosphate-free) is filtered onto a sintered-glass funnel and air dried at 120o for 24hours. [Lippin et al. J Am Chem Soc 76 2871 1954.] The purification can be carried out using a Soxhlet extractor (without cartridge), allowing longer extraction times. Treatment with conc H2SO4 instead of HCl has been used to remove reducing substances.