General Description
A gray lustrous powder. Used in powder metallurgy and as a catalyst in chemical manufacture.
Reactivity Profile
IRON, [POWDERED] is pyrophoric [Bretherick, 1979 p. 170-1]. A strong reducing agent and therefore incompatible with oxidizing agents. Burns in chlorine gas [Mellor 2, Supp. 1:380 1956]. Reacts with fluorine with incandescence [Mellor 13:314, 315, 1946-1947].
Air & Water Reactions
Highly flammable. May react with water to give off hydrogen, a flammable gas. The heat from this reaction may ignite the hydrogen.
Health Hazard
Fire may produce irritating and/or toxic gases. Contact may cause burns to skin and eyes. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished.
Description
Carbonyl iron is elemental iron produced by the decomposition of
iron pentacarbonyl as a dark gray powder. When viewed under a
microscope having a magnifying power of 500 diameters or
greater, it appears as spheres built up with concentric shells. It is
stable in dry air.
Physical properties
Pure iron is a silvery-white, hard, but malleable and ductile metal that can be worked andforged into many different shapes, such as rods, wires, sheets, ingots, pipes, framing, and soon. Pure iron is reactive and forms many compounds with other elements. It is an excellentreducing agent. It oxidizes (rusts) in water and moist air and is highly reactive with most acids,releasing hydrogen from the acid. One of its main properties is that it can be magnetized andretain a magnetic field.The iron with a valence of 2 is referred to as “ferrous” in compounds (e.g., ferrous chloride= FeCl2). When the valence is 3, it is called “ferric” (e.g., ferric chloride = FeCl3).Iron’s melting point is 1,535°C, its boiling point is 2,750°C, and its density is 7.873g/cm3.
Isotopes
There are 30 isotopes of iron ranging from Fe-45 to Fe-72. The following arethe four stable isotopes with the percentage of their contribution to the element’s naturalexistence on Earth: Fe-54 = 5.845%, Fe-56 = 91.72%, Fe-57 = 2.2%, and Fe-58 =0.28%. It might be noted that Fe-54 is radioactive but is considered stable because ithas such a long half-life (3.1×10+22 years). The other isotopes are radioactive and areproduced artificially. Their half-lives range from 150 nanoseconds to 1×105 years.
Origin of Name
The name “iron” or “iren” is Anglo-Saxon, and the symbol for iron (Fe)
is from ferrum, the Latin word for iron.
Occurrence
Iron is the fourth most abundant element in the Earth s crust (about 5%) and is the ninth most abundant element found in the sun and stars in the universe. The core of the Earth is believed to consist of two layers, or spheres, of iron. The inner core is thought to be molten iron and nickel mixture, and the outer core is a transition phase of iron with the molten magma of the Earth s mantle. Iron s two oxide compounds (ferrous(II) oxide FeO) and (ferric(III) oxide Fe2O3) are the third and seventh most abundant compounds found in the Earth s crust. The most common ore of iron is hematite that appears as black sand on beaches or black seams when exposed in the ground. Small amounts of iron and iron alloys with nickel and cobalt were found in meteorites (siderite) by early humans. This limited supply was used to shape tools and crude weapons. Even though small amounts of iron compounds and alloys are found in nature (iron is not found in its pure metallic state in nature), early humans did not know how to extract iron from ores until well after they knew how to smelt gold, tin, and copper ores. From these metals, they then developed bronze alloy thus the Bronze Age (about 8000 BCE). There are several grades of iron ores, including hematite (brown ferric oxide) and limonite (red ferric oxide). Other ores are pyrites, chromite, magnetite, siderite, and low-grade taconite. Magnetite (Fe3O4) is the magnetic iron mineral/ore found in South Africa, Sweden, and parts of the United States. The lodestone, a form of magnetite, is a natural magnet. Iron ores are found in many countries. Iron is found throughout most of the universe, in most of the stars, and in our sun, and it probably exists on the other planets of our solar system.
Characteristics
Iron is the only metal that can be tempered (hardened by heating, then quenching in wateror oil). Iron can become too hard and develop stresses and fractures. This can be corrected byannealing, a process that heats the iron again and then holds it at that temperature until thestresses are eliminated. Iron is a good conductor of electricity and heat. It is easily magnetized,but its magnetic properties are lost at high temperatures. Iron has four allotropic states. Thealpha form exists at room temperatures, while the other three allotropic forms exist at varyinghigher temperatures.Iron is the most important construction metal. It can be alloyed with many other metals tomake a great variety of specialty products. Its most important alloy is steel.An interesting characteristic of iron is that it is the heaviest element that can be formed byfusion of hydrogen in the sun and similar stars. Hydrogen nuclei can be “squeezed” in the sunto form all the elements with atomic numbers below cobalt (27Co), which includes iron. Itrequires the excess fusion energy of supernovas (exploding stars) to form elements with protonnumbers greater than iron (26Fe).
Preparation
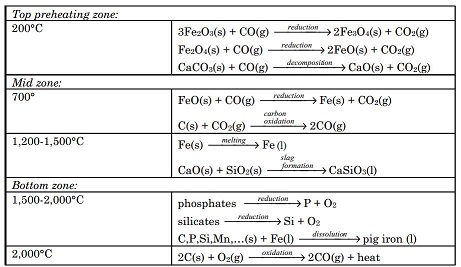
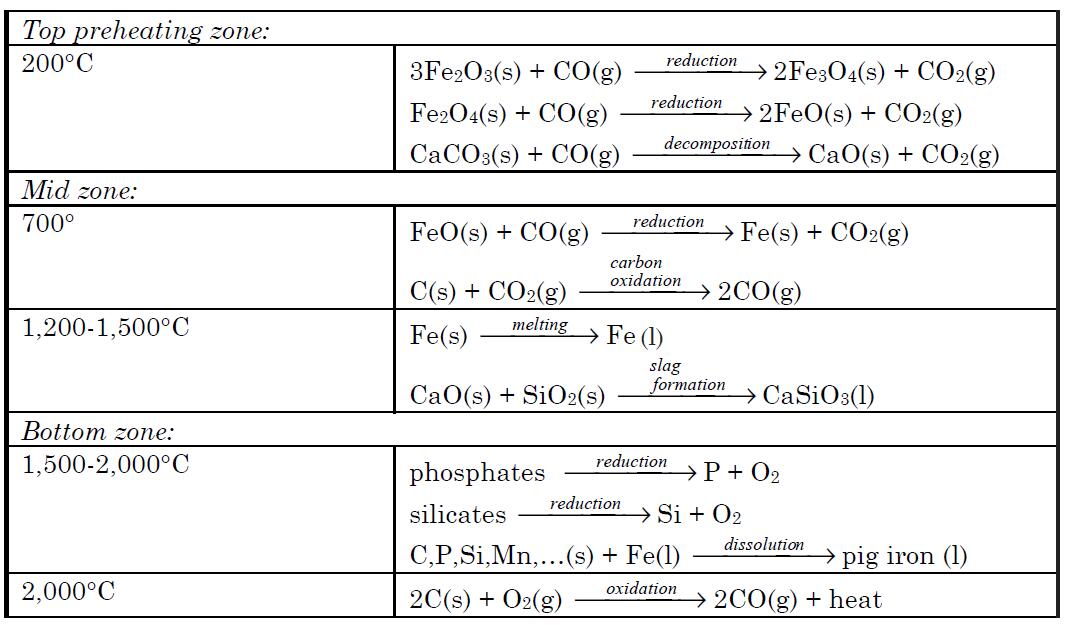
Most iron produced today is from its oxide minerals, hematite and magnetite. The process involves reducing mineral iron with carbon in a blast furnace. There are several types of blast furnaces which vary in design anddimensions. The overall processes, however, are more or less the same. Onesuch process is outlined below:
The mixture of ore, coke and limestone is fed into the blast furnace from thetop. The materials are preheated to about 200°C in the top most zone.Hematite is partially reduced to magnetite and then to FeO by the ascendingstream of carbon monoxide formed at the bottom and mid zones of the furnaceresulting from high temperature oxidation of carbon. The ferrous oxide FeOformed at the top zone is reduced to metallic iron at about 700°C in the midzone by carbon monoxide. A hot air blast at 900°C passes through the entirefurnace for a very short time (usually for a few seconds). This prevents any gassolid reaction product from reaching equilibrium. In the temperature zone 700to 1,200°C ferrous oxide is completely reduced to iron metal by carbon monox-ide. Also, more CO is formed by oxidation of carbon by carbon dioxide. Furtherdown the furnace at higher temperatures, around 1,500°C, iron melts, drippingdown into the bottom. Also, in this temperature zone acidic silica particlesreact with basic calcium oxide produced from the decomposition of limestone,producing calcium silicate. The molten waste calcium silicate also drips downinto the bottom. In the hottest zone of the blast furnace, between 1,500 to2,000°C, some carbon dissolves into the molten iron. Also at these temperatures any remaining silicates and phosphates are reduced to silicon and phosphorus, and dissolve into the molten iron. Additionally, other tract metals suchas manganese dissolve into the molten iron. The impure iron melt containingabout 3 to 4% carbon is called “pig iron”. At the bottom, the molten waste slagfloats over the impure pig iron melt that is heavier than the slag melt andimmiscible with it. Pig iron is separated from the slag and purified for makingdifferent types of steel. Chemical reactions and processes occurring in varioustemperature zones of blast furnace are summarized below:
Pig iron produced in the blast furnace is purified and converted to steel ina separate furnace, known as a basic-oxygen furnace. Jets of pure oxygen gasat high pressure are blown over and through the pig iron melt. Metal impurities are converted into oxides. Part of the dissolved carbon in the impure ironmelt is converted into carbon dioxide gas. Formation of SiO2, CO2,and othermetal oxides are exothermic reactions that raise the temperature to sustainthe melt. A lime flux (CaO) also is added into the melt, which converts silicainto calcium silicate, CaSiO3,and phosphorus into calcium phosphate,Ca3(PO4)2,forming a molten slag immiscible with molten steel. The lightermolten slag is decanted from the heavier molten steel.
Hazard
Iron dust from most iron compounds is harmful if inhaled and toxic if ingested. Iron dustand powder (even filings) are flammable and can explode if exposed to an open flame. Asmentioned, excessive iron in the diet may cause liver damage.
Biochem/physiol Actions
Carbonyl iron has a role as an absorber of microwave radiation. Carbonyl iron also exhibits shielding properties because of the low density and the connectivity among the fillers it provide. It is used in industries and in the synthesis of nitro-group-containing pharmaceutical ingredients.
Environmental Fate
Iron occurs rarely by itself in nature due to the ease with which
it forms compounds, especially in oxidation reactions. Many
iron compounds are water soluble, leading to potentially high
concentrations in water, especially in seawater. Iron is a necessary
component of all life and it is therefore taken up readily by
organisms from all sources.
Purification Methods
Clean it in conc HCl, rinse in de-ionised water, then reagent grade acetone and dry it under vacuum.
Structure and conformation
Two structural types of iron occur in the solid state. At room temperature iron has a
body-centered cubic lattice (the a form). At about 910°C the a form is transformed into
the γ allotrope which has a cubic close-packed structure. Around 1390°C a body-centred
cubic lattice is reformed—the δ form. Thus the allotropy of iron is unusual in that it can
exist with the same crystal form in two distinct temperature ranges which are separated by a
range within which a different form is stable. The a and ? forms have similar lattice parameters—
the differences between them being expected in view of thermal expansion which
increases the size of the unit cell of the δallotrope.
Toxicity evaluation
In some adults, iron overload can be the result of a genetic
defect (idiopathic hemochromatosis) that causes malfunction of the normal homeostasis mechanism and, in turn, excessive
absorption of iron. Iron overload can also be caused by too
many blood transfusions, which results in too much iron in the
various iron-containing organs.
Recently, it has been suggested that the presence of
increased transferrin concentrations in males is associated with
an increased number of heart attacks. This must be corroborated
by further research. Excess iron can lead to diabetes
mellitus, faulty liver functions, and endocrine disturbance.
Iron is a catalyst for oxidative damage leading to lipid peroxidation.
The latest hypotheses link peroxidation to heart
disease, cancer, and accelerated aging. Iron is involved in the
Fenton reaction, which catalyzes the formation of free radicals
that cause excessive damage to cells and their components.